Risperdal Consta 37.5 Mg Powder And Solvent For Prolonged-Release Suspension For Intramuscular Injec
Page 4 Page 1
Page 4 Page 1
• Inflammation of the pancreas, a blockage in the bowels
• Very hard stool
• Rash on skin-related to drug
• Hives (or "nettle rash"), thickening of skin, dandruff, skin disorder, skin lesion
• Breakdown of muscle fibers and pain in muscles (rhabdomyolysis)
• Abnormal posture
• Breast enlargement, discharge from the breasts
• Decreased body temperature, discomfort
• Yellowing of the skin and the eyes (jaundice)
• Severe allergic reaction characterised by fever, swollen mouth, face, lip or tongue, shortness of breath, itching, skin rash and sometimes drop in blood pressure
• Dangerously excessive intake of water
• Increased insulin (a hormone that controls blood sugar levels) in your blood
• Blood vessel problems in the brain
• Unresponsive to stimuli
• Coma due to uncontrolled diabetes
• Sudden loss of vision or blindness
• Glaucoma (increased pressure within the eyeball), eyelid margin crusting
• Flushing, swollen tongue
• Chapped lips
• Priapism (a prolonged penile erection that may require surgical treatment)
• Enlargement of the glands in your breasts
• A decrease in body temperature, coldness in arms and legs
• Symptoms of drug withdrawal.
Very rare side effects (may affect up to 1 in
10,000 people)
• Life-threatening complications of uncontrolled diabetes
• Serious allergic reaction with swelling that may involve the throat and lead to difficulty breathing
• Lack of bowel muscle movement that causes blockage.
The following side effect has been seen with the use of another medicine called paliperidone that is very similar to risperidone, so these can also be expected with Risperdal Consta: Rapid heartbeat upon standing.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme via www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Risperdal Consta
Keep out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton. The expiry date refers to the last day of that month.
Store in refrigerator (2-8°C).
Protect from light.
If refrigeration is unavailable; store at a temperature not exceeding 25°C for no more than 7 days prior to administration.
After reconstitution: Do not store above 25°C and use within 6 hours.
Store in the original package.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information
What Risperdal Consta contains
Each Risperdal Consta powder and solvent for prolonged-release suspension for injection contains either 25 mg, 37.5 mg or 50 mg of risperidone.
Other ingredients are: poly-(D,1-lactide-co-glycolide).
Solvent (solution)
Polysorbate 20, carmellose sodium, sodium phosphate dihydrate, Citric acid anhydrous, Sodium chloride, Sodium hydroxide, Water for injection.
What Risperdal Consta looks like and contents of the pack
• One vial containing the powder for prolonged-release suspension for injection (within this powder is the active substance, risperidone). One syringe filled with 2 ml of clear, colourless liquid to be added to the powder for prolonged-release suspension for injection.
• One Alaris™ SmartSite Needle-Free Vial for reconstitution
• Two needles for intramuscular injection (a 21G UTW 1-inch (0.8 mm x 25 mm) safety needle with Needle-Protection safety device for deltoid administration and a 20G TW 2-inch (0.9 mm x 50 mm) safety needle with Needle-protection safety device for gluteal administration).
Manufacturer and Product Licence Holder
This product is manufactured by Janssen Pharmaceutica NV, Turnhoutseweg 30, B-2340, Beerse, Belgium. It is procured from within the EU by the Product Licence Holder: Swinghope Limited, Brandon House, Marlowe Way, Croydon CR0 4XS, UK.
PL No:10380/1599 Risperdal Consta 25 mg PL No:10380/1596 Risperdal Consta 37.5 mg PL No:10380/1600 Risperdal Consta 50 mg
Leaflet revision date: 09/06/2016
Risperdal Consta® is registered trademark of Johnson & Johnson., USA.
POM
T06614
Risperdal® Consta® 25, 37.5 and 50 mg powder and solvent for prolonged-release suspension for intramuscular injection
(risperidone)
Patient Information Leaflet
This medicine is available using the above name but will be referred to as Risperdal Consta throughout the Patient Information Leaflet.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Risperdal Consta is and what it is used for
2. What you need to know before you use Risperdal Consta
3. How to use Risperdal Consta
4. Possible side effects
5. How to store Risperdal Consta
6. Contents of the pack and other information
1. What Risperdal Consta is and what it is used for
Risperdal Consta belongs to a group of medicines called ‘anti-psychotics'.
Risperdal Consta is used to maintain the treatment of schizophrenia, where you may see, hear or feel things that are not there, believe things that are not true or feel unusually suspicious, or confused.
Risperdal Consta is intended for patients who are currently treated with oral (e.g. tablets, capsules) antipsychotics.
Risperdal Consta can help alleviate the symptoms of your disease and stop your symptoms from coming back.
2. What you need to know before you use Risperdal Consta
Do not use Risperdal Consta
• If you are allergic to risperidone or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
• If you have never taken any form of Risperdal, you should begin with oral Risperdal before beginning treatment with Risperdal Consta.
Talk to your doctor or pharmacist before using Risperdal Consta if:
• You have a heart problem. Examples include an irregular heart rhythm or if you are prone to low blood pressure or if you are using medicines for your blood pressure. Risperdal Consta may cause low blood pressure. Your dose may need to be adjusted.
• You know of any factors which would favour you having a stroke, such as high blood pressure, cardiovascular disorder or circulation disorders of the brain
• You have ever experienced involuntary movements of the tongue, mouth and face
• You have ever had a condition whose symptoms include high temperature, muscle stiffness, sweating or a lowered level of consciousness (also known as Neuroleptic Malignant Syndrome)
• You have Parkinson's disease or dementia
• You know that you have had low levels of white blood cells in the past (which may or may not have been caused by other medicines)
• You are diabetic
• You have epilepsy
• You are a man and have ever had a prolonged or painful erection.
• You have difficulty controlling body temperature or overheating
• You have kidney problems
• You have liver problems
• You have an abnormally high level of the hormone prolactin in your blood or if you have a possible prolactin-dependent tumour
• You or someone else in your family has a history of blood clots, as medicines like these have been associated with formation of blood clots.
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using Risperdal or Risperdal Consta.
As dangerously low numbers of a certain type of white blood cell needed to fight infection in your blood has been seen very rarely with patients using Risperdal Consta, your doctor may check your white blood cell counts.
Even if you have previously tolerated oral risperidone, rarely allergic reactions occur after receiving injections of Risperdal Consta. Seek medical attention right away if you experience a rash, swelling of your throat, itching, or problems breathing as these may be signs of a serious allergic reaction.
Risperdal Consta may cause you to gain weight. Significant weight gain may adversely affect your health. Your doctor should regularly measure your body weight.
As diabetes mellitus or worsening of pre-existing diabetes mellitus have been seen with patients taking Risperdal, your doctor should check for signs of high blood sugar. In patients with pre-existing diabetes mellitus blood glucose should be monitored regularly.
Risperdal Consta commonly raises levels of a hormone called "prolactin". This may cause side effects such as menstrual disorders or fertility problems in women, breast swelling in men (see Possible side effects). If such side effects occur, evaluation of the prolactin level in the blood is recommended.
During an operation on the eye for cloudiness of the lens (cataract), the pupil (the black circle in the middle of your eye) may not increase in size as needed. Also, the iris (the coloured part of the eye) may become floppy during surgery and that may lead to eye damage. If you are planning to have an operation on your eye, make sure you tell your eye doctor that you are using this medicine.
Elderly with dementia
Risperdal Consta is not for use in elderly people with dementia.
Medical treatment should be sought straight away if you or your carer notice a sudden change in your mental state or sudden weakness or numbness of your face, arms or legs, especially on one side, or slurred speech, even for a short period of time. These may be signs of a stroke.
People with kidney or liver problems
Although oral risperidone has been studied, Risperdal Consta has not been studied in patients with kidney or liver problems. Risperdal Consta should be administered with caution in this patient group.
Other medicines and Risperdal Consta
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
It is especially important to talk to your doctor or pharmacist if you are taking any of the following
• Medicines that work on your brain to help you calm down (benzodiazepines), or some medicines for pain (opiates), medicines for allergy (some antihistamines), as risperidone may increase the sedative effect of all of these.
• Medicines that may change the electrical activity of your heart, such as medicines for malaria, heart rhythm problems, allergies (anti-histamines), some antidepressants or other medicines for mental problems
• Medicines that cause a slow heart beat
• Medicines that cause low blood potassium (such as certain diuretics)
• Medicines for Parkinson's disease (such as levodopa)
• Medicines to treat raised blood pressure. Risperdal Consta can lower blood pressure
• Water tablets (diuretics) used for heart problems or swelling of parts of your body due to a build up of too much fluid (such as furosemide or chlorothiazide). Risperdal Consta taken by itself or with furosemide, may have an increased risk of stroke or death in elderly people with dementia.
The following medicines may reduce the effect of risperidone
• Rifampicin (a medicine for treating some infections)
• Carbamazepine, phenytoin (medicines for epilepsy)
• Phenobarbital
If you start or stop taking such medicines you may need a different dose of risperidone.
The following medicines may increase the effect of risperidone
• Quinidine (used for certain types of heart disease)
• Antidepressants such as paroxetine, fluoxetines, tricyclic antidepressants
• Medicines known as beta-blockers (used to treat high blood pressure)
• Phenothiazines (such as medicines used to treat psychosis or to calm down)
• Cimetidine, ranitidine (blockers of the acidity of stomach)
• Itraconazole and ketoconazole (medicines for treating fungal infections)
• Certain medicines used in the treatment of HIV/AIDS, such as ritonavir
• Verapamil, a medicine used to treat high blood pressure and/or abnormal heart rhythm
• Sertraline and fluvoxamine, medicines used to treat depression and other psychiatric disorders.
If you start or stop taking such medicines you may need a different dose of risperidone
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using Risperdal Consta.
Risperdal Consta with food, drink and alcohol
You should avoid drinking alcohol when using Risperdal Consta.
Pregnancy, breast-feeding and fertility
• If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. Your doctor will decide if you can use it.
• The following symptoms may occur in newborn babies, of mothers that have used Risperdal Consta in the last trimester (last three months of their pregnancy): shaking, muscle stiffness, and/or weakness, sleepiness, agitation, breathing problems, and difficulty in feeding. If your baby develops any of these symptoms you may need to contact your doctor.
• Risperdal Consta can raise your levels of a hormone called "prolactin" that may impact fertility (see Possible side effects).
Driving and using machines
Dizziness, tiredness, and vision problems may occur during treatment with Risperdal Consta. Do not drive or use any tools or machines without talking to your doctor first.
Risperdal Consta contains less than 1 mmol sodium (23 mg) per dose, i.e., essentially ‘sodium-free'.
3. How to use Risperdal Consta
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Risperdal Consta is given as an intramuscular injection either in the arm or buttock every two weeks, administered by a health care professional. Injections should be alternated between the right and left sides, and should not be given intravenously.
The recommended dose is as follows Adults
Starting dose
If your daily dose of oral (e.g. tablets)
risperidone was 4 mg or less for the last two weeks, your starting dose should be 25 mg Risperdal Consta.
If your daily dose of oral (e.g. tablets)
risperidone was more than 4 mg for the last two weeks, you may be given 37.5 mg Risperdal Consta as a starting dose.
If you are currently treated with other oral antipsychotics than risperidone, your starting dose of Risperdal Consta will depend on your current treatment. Your doctor will choose Risperdal Consta 25 mg or 37.5 mg.
Your doctor will decide on the dose of Risperdal Consta that is right for you.
Maintenance dose
• The usual dose is 25 mg every two weeks as an injection.
• A higher dose of 37.5 or 50 mg may be necessary. Your doctor will decide on the dose of Risperdal Consta that is right for you.
• Your doctor may prescribe oral Risperdal for the first three weeks following your first injection.
If you are given more Risperdal Consta than you should
• People who have been given more Risperdal Consta than they should have experienced the following symptoms: sleepiness, tiredness, abnormal body movements, problems with standing and walking, dizziness from low blood pressure, and abnormal heart beats. Cases of abnormal electrical conduction in the heart and convulsion have been reported.
• See a doctor right away.
If you stop using Risperdal Consta
You will lose the effects of the medicine. You should not stop this medicine unless told to do so by your doctor as your symptoms may return. Be sure not to miss your appointments when you are supposed to receive your injections every two weeks. If you cannot keep your appointment, be sure to contact your doctor right away to discuss another date when you can come in for your injection. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Use in children and adolescents
Risperdal Consta is not for people who are under 18 years old.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately if you:
• Experience blood clots in the veins, especially in the legs (symptoms include swelling, pain, and redness in the leg), which may travel through blood vessels to the lungs causing chest pain and difficulty breathing. If you notice any of these symptoms seek medical advice immediately
• Have dementia and experience a sudden change in your mental state or sudden weakness or numbness of your face, arms or legs, especially on one side, or slurred speech, even for a short period of time. These may be signs of a stroke
• Experience fever, muscle stiffness, sweating or a lowered level of consciousness (a disorder called “Neuroleptic Malignant Syndrome”). Immediate medical treatment may be needed
• Are a man and experience prolonged or painful erection. This is called priapism. Immediate medical treatment may be needed
• Experience involuntary rhythmic movements of the tongue, mouth and face. Withdrawal of risperidone may be needed
• Experience severe allergic reaction characterised by fever, swollen mouth, face, lip or tongue, shortness of breath, itching, skin rash or drop in blood pressure. Even if you have previously tolerated oral risperidone, rarely allergic reactions occur after receiving injections of Risperdal Consta.
The following side effects may happen:
Very common side effects (may affect more
than 1 in 10 people)
• Common cold symptoms
• Difficulty falling or staying asleep
• Depression, anxiety
• Parkinsonism: This condition may include: slow or impaired movement, sensation of stiffness or tightness of the muscles (making your movements jerky), and sometimes even a sensation of movement "freezing up" and then restarting. Other signs of parkinsonism include a slow shuffling walk, a tremor while at rest, increased saliva and/or drooling, and a loss of expression on the face.
• Headache.
Common side effects (may affect up to 1 in
10 people)
• Pneumonia, infection of the chest (bronchitis), sinus infection
• Urinary tract infection, feeling like you have the flu, anemia
• Raised levels of a hormone called "prolactin" found in a blood test (which may or may not cause symptoms). Symptoms of high prolactin occur uncommonly and may include in men breast swelling, difficulty in getting or maintaining erections, decreased sexual desire or other sexual dysfunction. In women they may include breast discomfort, leakage of milk from the breasts, missed menstrual periods, or other problems with your cycle or fertility problems.
• High blood sugar, weight gain, increased appetite, weight loss, decreased appetite
• Sleep disorder, irritability, decreased sexual drive, restlessness, feeling sleepy, or less alert
• Dystonia. This is a condition involving slow or sustained involuntary contraction of muscles. While it can involve any part of the body (and may result in abnormal posture), dystonia often involves muscles of the face, including abnormal movements of the eyes, mouth, tongue or jaw.
• Dizziness
• Dyskinesia: This is a condition involving involuntary muscle movements, and can include repetitive, spastic or writhing movements, or twitching.
• Tremor (shaking)
• Blurry vision
• Rapid heart rate
• Low blood pressure, chest pain, high blood pressure
• Shortness of breath, sore throat, cough, stuffy nose
• Abdominal pain, abdominal discomfort, vomiting, nausea, stomach or intestinal infection, constipation, diarrhea, indigestion, dry mouth, toothache
• Rash
• Muscle spasms, bone or muscle ache, back pain, joint pain
• Incontinence (lack of control) of urine
• Erectile dysfunction
• Loss of menstrual periods
• Leakage of milk from the breasts
• Swelling of the body, arms or legs, fever, weakness, fatigue (tiredness)
• Pain
• A reaction at the injection site, including itching, pain or swelling
• Increased liver transaminases in your blood, increased GGT (a liver enzyme called gamma-glutamyltransferase) in your blood
• Fall.
Uncommon side effects (may affect up to 1 in
100 people)
• Infection of the breathing passages, bladder infection, ear infection
• eye infection, tonsillitis, fungal infection of the nails, infection of the skin, an infection confined to a single area of skin or part of the body, viral infection, skin inflammation caused by mites, abscess under the skin
• White blood cell count decreased, decrease in platelets (blood cells that help you stop bleeding), decrease in red blood cells
• Allergic reaction
• Sugar in the urine, diabetes or worsening of diabetes
• Loss of appetite resulting in malnutrition and low body weight
• High blood triglycerides (a fat), increased cholesterol in your blood
• Elated mood (mania), confusion, Inability to reach orgasm, nervousness, nightmares
• Tardive dyskinesia (twitching or jerking movements that you cannot control in your face, tongue, or other parts of your body). Tell your doctor immediately if you experience involuntary rhythmic movements of the tongue, mouth and face. Withdrawal of Risperdal Consta may be needed.
• Sudden loss of blood supply to brain (stroke or "mini" stroke)
• Loss of consciousness, convulsion (fits), fainting
• A restless urge to move parts of your body, balance disorder, abnormal coordination, dizziness upon standing, disturbance in attention, problems with speech, loss or abnormal sense of taste, reduced sensation of skin to pain and touch, a sensation of tingling, pricking, or numbness of skin
• Eye infection or "pink eye", dry eye, increased tears, redness of the eyes
• Sensation of spinning (vertigo), ringing in the ears, ear pain
• Atrial fibrillation (an abnormal heart rhythm), an interruption in conduction between the upper and lower parts of the heart, abnormal electrical conduction of the heart, prolongation of the QT interval from your heart, slow heart rate, abnormal electrical tracing of the heart (electrocardiogram or ECG), a fluttering or pounding feeling in your chest (palpitations)
• Low blood pressure upon standing (consequently, some people using Risperdal Consta may feel faint, dizzy, or may pass out when they stand up or sit up suddenly)
• Fast, shallow breathing, congestion of breathing passages, wheezing, nosebleeds
• Stool incontinence, difficulty swallowing, excessive passing of gas or wind
• Itching, hair loss, eczema, dry skin, skin redness, skin discoloration, acne, flaky, itchy scalp or skin
• An increase of CPK (creatine
phosphokinase) in your blood, an enzyme which is sometimes released with muscle breakdown
• Joint stiffness, joint swelling, muscle
weakness, neck pain
• Frequent passing of urine, inability to pass urine, pain when passing urine
• Ejaculation disorder, a delay in menstrual
periods, missed menstrual periods or other problems with your cycle (females),
development of breasts in men, sexual dysfunction, breast pain, breast discomfort, vaginal discharge
• Swelling of the face, mouth, eyes, or lips
• Chills, an increase in body temperature
• A change in the way you walk
• Feeling thirsty, feeling unwell, chest discomfort, feeling "out of sorts"
• Hardening of the skin
• Increased liver enzymes in your blood
• Procedural pain.
Rare side effects (may affect up to 1 in 1,000 people)
• Decrease in the type of white blood cells that help to protect you against infection
• Inappropriate secretion of a hormone that controls urine volume
• Low blood sugar
• Excessive drinking of water
• Lack of emotion
• Neuroleptic malignant syndrome (confusion, reduced or loss of consciousness, high fever, and severe muscle stiffness)
• Low level of consciousness
• Shaking of the head
• Problems with movement of your eyes, eye rolling, oversensitivity of the eyes to light
• Eye problems during cataract surgery. During cataract surgery, a condition called intraoperative floppy iris syndrome (IFIS) can happen if you use or have used Risperdal Consta. If you need to have cataract surgery, be sure to tell your eye doctor if you use or have used this medicine.
• Irregular heart beat
• Dangerously low numbers of a certain type of white blood cell needed to fight infection in your blood, increase in eosinophils (a type of white blood cell) in your blood, blood clot in the legs, blood clot in the lungs
• Trouble breathing during sleep (sleep apnea)
• Pneumonia caused by inhaling food, lung congestion, crackly lung sounds, voice disorder breathing passage disorder
T06614
T06615
IMPORTANT INFORMATION FOR HEALTHCARE PROFESSIONALS
Risperdal® Consta® 25, 37.5 and 50 mg powder and solvent for prolonged-release suspension for intramuscular
injection
(risperidone)
RISPERDAL® CONSTA® requires close attention to these step-by-step Instructions for Use to help ensure successful administration.
Use components provided
The components in this dose pack are specifically designed for use with RISPERDAL® CONSTA®. RISPERDAL® CONSTA® must be reconstituted only in the diluent supplied in the dose pack.
Do not substitute ANY components of the dose pack.
Do not store suspension after reconstitution
Administer dose as soon as possible after reconstitution to avoid settling.
Proper dosing
The entire contents of the vial must be administered to ensure intended dose of RISPERDAL® CONSTA® is delivered.
SINGLE-USE DEVICE
Do not reuse. Medical devices require specific material characteristics to perform as intended. These characteristics have been verified for single use only. Any attempt to re-process the device for subsequent re-use may adversely affect the integrity of the device or lead to deterioration in performance.
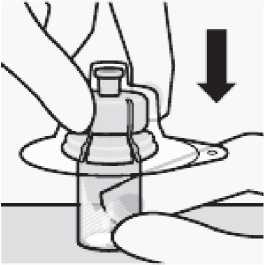
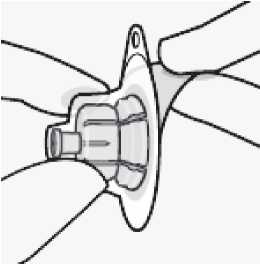
Connect vial adapter to vial
Place vial on a hard surface and hold by the base. Center vial adapter over the grey rubber stopper. Push vial adapter straight down onto vial top until it snaps securely into place.
Do not place vial adapter on at an angle or diluent may leak upon transfer to the vial.
X Incorrect
Connect prefilled syringe to vial adapter
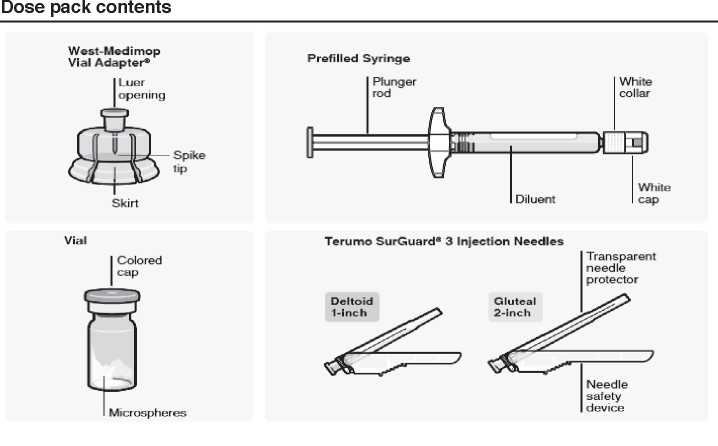
Step 1. Assemble componets
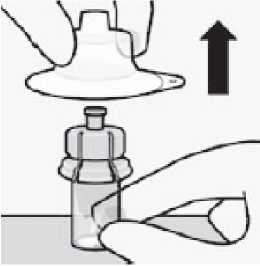
Remove sterile blister
Remove vial adaptor from sterile blister only when you are ready to remove the white cap from the prefilled syringe.
Keep vial vertical to prevent leakage.
Hold base of vial and pull up on the sterile blister to remove.
Do not shake.

Do not touch exposed luer opening on vial adapter. This will result in contamination.
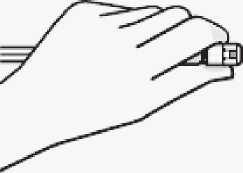
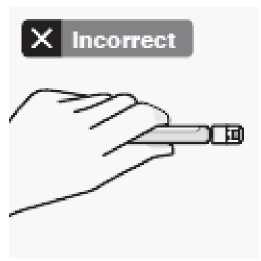
Use proper grip
Hold by white collar at the tip of the syringe.
Do not hold syringe by the glass barrel during assembly.
Take out dose pack
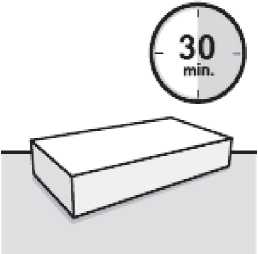
Wait 30 minutes
Remove dose pack from the refrigerator and allow to sit at room temperature for at least 30 minutes before reconstituting.
Do not warm any other way.
Connect vial adapter to vial
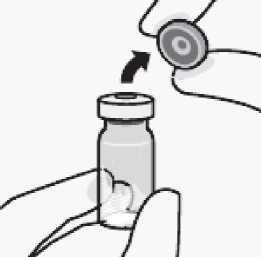
Remove cap from vial Flip off coloured cap from vial.
Wipe top of the grey stopper with an alcohol swab Allow to air dry.
Do not remove grey rubber stopper.
SNAP!
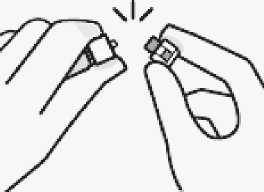
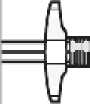
Remove cap
Holding the white collar, snap off the white cap. Do not twist or cut off the white cap.
Do not touch syringe tip. This will result in contamination.
The broken-off cap can be discarded.
Prepare vial adapter
Hold sterile blister as shown.
Peel back and remove paper backing.
Do not remove vial adapter from blister.
Do not touch spike tip at any time. This will result in contamination.
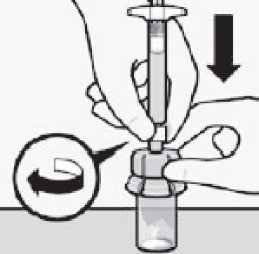
Connect syringe to vial adapter
Hold vial adapter by skirt to keep stationary. Hold syringe by white collar then insert tip into the luer opening of the vial adapter.
Do not hold the glass syringe barrel.
This may cause the white collar to loosen or detach. Attach the syringe to the vial adapter with a firm clockwise twisting motion until it feels snug.
Do not over-tighten. Over-tightening may cause the syringe tip to break.
Resuspend microspheres
Fully remove the blister pouch.
Just before injection, shake syringe vigorously again, as some settling will have occurred.
Step 4. Inject dose
Step 2. Reconstitute microspheres
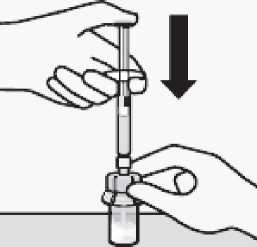
Inject diluent
Inject entire amount of diluent from syringe into the vial.
Vial contents will now be under pressure.
Keep holding the plunger rod down with thumb.

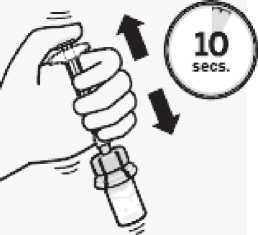
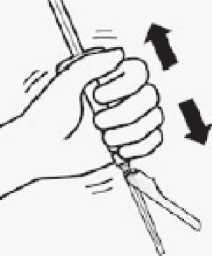
Suspend microspheres in diluent
Continuing to hold down the plunger rod, shake vigorously for at least 10 seconds, as shown.
Check the suspension. When properly mixed, the suspension appears uniform, thick and milky in colour.
Microspheres will be visible in the liquid. Immediately proceed to the next step so suspension does not settle.
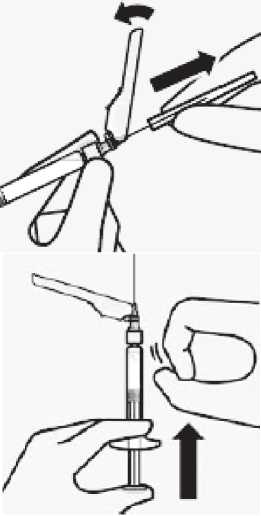
Remove air bubbles
Hold syringe upright and tap gently to make any air bubbles rise to the top.
Slowly and carefully press plunger rod upward to remove air.
Remove transparent needle protector
Move the needle safety device back towards the syringe, as shown. Then hold white collar on syringe and carefully pull the transparent needle protector straight off.
Do not twist transparent needle protector, as the luer connection may loosen.
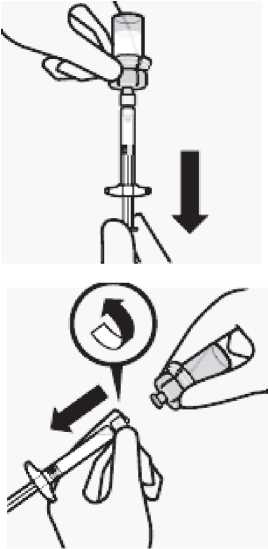
Transfer suspension to syringe
Invert vial completely. Slowly pull plunger rod down to withdraw entire contents from the vial into the syringe.
Inject
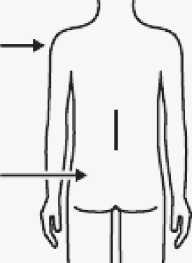
Immediately inject entire contents of syringe intramuscularly (IM) into the gluteal or deltoid muscle of the patient.
Gluteal injection should be made into the upper-outer quadrant of the gluteal area.
Do not administer intravenously.
Remove vial adapter
Hold white collar on the syringe and unscrew from vial adapter.
Tear section of the vial label at the perforation.
Apply detached label to the syringe for identification purposes.
Discard both vial and vial adapter appropriately.
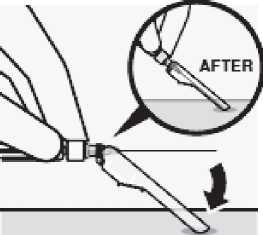
Secure needle in safety device
Using one hand, place needle safety device at a 45 degree angle on a hard, flat surface. Press down with a firm, quick motion until needle is fully engaged in safety device.
Avoid needle stick injury:
Do not use two hands.
Do not intentionally disengage or mishandle the needle safety device.
Do not attempt to straighten the needle or engage the safety device if the needle is bent or damaged.
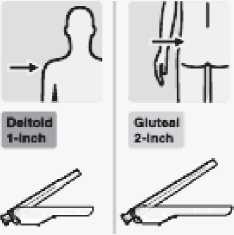
Select appropriate needle
Choose needle based on injection location (gluteal or deltoid).
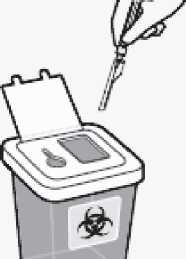
Properly dispose of needles
Check to confirm needle safety device is fully engaged.
Discard in an approved sharps container.
Also discard the unused needle provided in the dose pack.
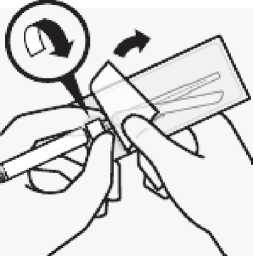
Attach needle
Peel blister pouch open part way and use to grasp the base of the needle, as shown.
Holding the white collar on the syringe, attach syringe to needle luer connection with a firm clockwise twisting motion until snug.
Do not touch needle luer opening. This will result in contamination.
Manufacturer and Product Licence Holder
This product is manufactured by Janssen Pharmaceutica NV, Turnhoutseweg 30, B-2340, Beerse, Belgium. It is procured from within the EU by the Product Licence Holder: Swinghope Limited, Brandon House, Marlowe Way, Croydon CR0 4XS, UK.
POM
PL No: 10380/1599 Risperdal Consta 25 mg
PL No: 10380/1596 Risperdal Consta 37.5 mg
PL No: 10380/1600 Risperdal Consta 50 mg
Leaflet revision date: 09/06/2016
Risperdal Consta® is registered trademark of Johnson & Johnson., USA.
T06615