Risperdal Consta 37.5Mg Powder And Solvent For Prolonged-Release Suspension For Intramuscular Inject
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
RISPERDAL CONSTA 37.5 mg powder and solvent for prolonged-release suspension for intramuscular injection
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
1 vial contains 37.5 mg risperidone.
1 ml reconstituted suspension contains 18.75 mg of risperidone.
Excipients with known effect: 1 ml reconstituted suspension contains 3 mg sodium.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Powder and solvent for prolonged-release suspension for injection.
Vial with powder
White to off-white free flowing powder.
Pre-filled syringe of solvent for reconstitution Clear, colourless aqueous solution.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
RISPERDAL CONSTA is indicated for the maintenance treatment of schizophrenia in patients currently stabilised with oral antipsychotics.
4.2 Posology and method of administration
Posology
Adults
Starting dose:
For most patients the recommended dose is 25 mg intramuscular every two weeks. For those patients on a fixed dose of oral risperidone for two weeks or more, the following conversion scheme should be considered. Patients treated with a dosage of 4 mg or less oral risperidone
should receive 25 mg RISPERDAL CONSTA, while patients treated with higher oral doses should be considered for the higher RISPERDAL CONSTA dose of 37.5 mg.
Where patients are not currently taking oral risperidone, the oral pre-treatment dosage should be considered when choosing the I.M. starting dose. The recommended starting dose is 25 mg RISPERDAL CONSTA every two weeks. Patients on higher dosages of the used oral antipsychotic should be considered for the higher RISPERDAL CONSTA dose of 37.5 mg.
Sufficient antipsychotic coverage with oral risperidone or the previous antipsychotic should be ensured during the three-week lag period following the first RISPERDAL CONSTA injection (see section 5.2).
RISPERDAL CONSTA should not be used in acute exacerbations of schizophrenia without ensuring sufficient antipsychotic coverage with oral risperidone or the previous antipsychotic during the three-week lag period following the first RISPERDAL CONSTA injection.
Maintenance dose:
For most patients the recommended dose is 25 mg intramuscular every two weeks. Some patients may benefit from the higher doses of 37.5 mg or 50 mg. Upward dosage adjustment should not be made more frequently than every 4 weeks. The effect of this dose adjustment should not be anticipated earlier than 3 weeks after the first injection with the higher dose. No additional benefit was observed with 75 mg in clinical trials. Doses higher than 50 mg every 2 weeks are not recommended.
Elderly
No dose adjustment is required. The recommended dose is 25 mg intramuscularly every two weeks. Where patients are not currently taking oral risperidone, the recommended dose is 25 mg RISPERDAL CONSTA every two weeks. For those patients on a fixed dose of oral risperidone for two weeks or more, the following conversion scheme should be considered. Patients treated with a dosage of 4 mg or less oral risperidone should receive 25 mg RISPERDAL CONSTA, while patients treated with higher oral doses should be considered for the higher RISPERDAL CONSTA dose of 37.5 mg.
Sufficient antipsychotic coverage should be ensured during the three-week lag period following the first RISPERDAL CONSTA injection (see section 5.2). RISPERDAL CONSTA clinical data in elderly are limited. RISPERDAL CONSTA should be used with caution in elderly.
Hepatic and renal impairment
RISPERDAL CONSTA has not been studied in hepatically and renally impaired patients.
If hepatically or renally impaired patients require treatment with RISPERDAL CONSTA, a starting dose of 0.5 mg twice daily oral risperidone is recommended during the first week. The second week 1 mg twice daily or 2 mg once daily can be given. If an oral total daily dose of at least 2 mg is well tolerated, an injection of 25 mg RISPERDAL CONSTA can be administered every 2 weeks.
Sufficient antipsychotic coverage should be ensured during the three-week lag period following the first RISPERDAL CONSTA injection (see section 5.2).
Paediatric population
The safety and efficacy of RISPERDAL CONSTA in children below 18 years of age have not been established. No data are available.
Method of administration
RISPERDAL CONSTA should be administered every two weeks by deep intramuscular deltoid or gluteal injection using the appropriate safety needle. For deltoid administration, use the 1-inch needle alternating injections between the two arms. For gluteal administration, use the 2-inch needle alternating injections between the two buttocks. Do not administer intravenously (see sections 4.4 and 6.6).
For instructions on reconstitution of the medicinal product before administration, see section 6.6.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
4.4 Special warnings and precautions for use
For risperidone-naive patients, it is recommended to establish tolerability with oral risperidone prior to initiating treatment with RISPERDAL CONSTA (see section 4.2).
Elderly with dementia
RISPERDAL CONSTA has not been studied in elderly patients with dementia, hence it is not indicated for use in this group of patients. RISPERDAL CONSTA is not licensed for the treatment of dementia-related behavioural disturbances.
Increased mortality in elderly with dementia
In a meta-analysis of 17 controlled trials of atypical antipsychotics, including oral RISPERDAL, elderly patients with dementia treated with atypical antipsychotics have an increased mortality compared to placebo. In placebo-controlled trials with oral RISPERDAL in this population, the incidence of mortality was 4.0% for RISPERDAL-treated patients compared to 3.1% for placebo-treated patients. The odds ratio (95% exact confidence interval) was 1.21 (0.7; 2.1). The mean age (range) of patients who died was 86 years (range 67-100). Data from two large observational studies showed that elderly people with dementia who are treated with conventional antipsychotics are also at a small increased risk of death compared with those who are not treated. There are insufficient data to give a firm estimate of the precise magnitude of the risk and the cause of the increased risk is not known. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear.
Concomitant use with furosemide
In the oral RISPERDAL placebo-controlled trials in elderly patients with dementia, a higher incidence of mortality was observed in patients treated with furosemide plus risperidone (7.3%; mean age 89 years, range 75-97) when compared to patients treated with risperidone alone (3.1%; mean age 84 years, range 70-96) or furosemide alone (4.1%; mean age 80 years, range 67-90). The increase in mortality in patients treated with furosemide plus risperidone was observed in two of the four clinical trials. Concomitant use of risperidone with other diuretics (mainly thiazide diuretics used in low dose) was not associated with similar findings.
No pathophysiological mechanism has been identified to explain this finding, and no consistent pattern for cause of death observed. Nevertheless, caution should be exercised and the risks and benefits of this combination or co-treatment with other potent diuretics should be considered prior to the decision to use. There was no increased incidence of mortality among patients taking other diuretics as concomitant treatment with risperidone. Irrespective of treatment, dehydration was an overall risk factor for mortality and should therefore be carefully avoided in elderly patients with dementia.
Cerebrovascular adverse events (CVAE)
An approximately 3-fold increased risk of cerebrovascular adverse events have been seen in randomised placebo-controlled clinical trials in the dementia population with some atypical antipsychotics. The pooled data from six placebo-controlled studies with RISPERDAL in mainly elderly patients (> 65 years of age) with dementia showed that CVAEs (serious and non-serious, combined) occurred in 3.3% (33/1009) of patients treated with risperidone and 1.2% (8/712) of patients treated with placebo. The odds ratio (95% exact confidence interval) was 2.96 (1.34; 7.50). The mechanism for this increased risk is not known. An increased risk cannot be excluded for other antipsychotics or other patient populations. RISPERDAL CONSTA should be used with caution in patients with risk factors for stroke.
Orthostatic hypotension
Due to the alpha-blocking activity of risperidone, (orthostatic) hypotension can occur, especially during initiation of treatment. Clinically significant hypotension has been observed post-marketing with concomitant use of risperidone and antihypertensive treatment. Risperidone should be used with caution in patients with known cardiovascular disease (e.g. heart failure, myocardial infarction, conduction abnormalities, dehydration, hypovolemia, or cerebrovascular disease). The risk/benefit of further treatment with RISPERDAL CONSTA should be assessed if clinically relevant orthostatic hypotension persists.
Leukopenia, neutropenia, and agranulocytosis
Events of leucopenia, neutropenia and agranulocytosis have been reported with antipsychotic agents, including RISPERDAL CONSTA. Agranulocytosis has been reported very rarely (< 1/10,000 patients) during post-marketing surveillance.
Patients with a history of a clinically significant low white blood cell count (WBC) or a drug-induced leukopenia/neutropenia should be monitored during the first few months of therapy and discontinuation of RISPERDAL CONSTA should be considered at the first sign of a clinically significant decline in WBC in the absence of other causative factors.
Patients with clinically significant neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count < 1 X 109/L) should discontinue RISPERDAL CONSTA and have their WBC followed until recovery.
Tardive dyskinesia/extrapyramidal symptoms (TD/EPS)
Medicines with dopamine receptor antagonistic properties have been associated with the induction of tardive dyskinesia characterised by rhythmical involuntary movements. predominantly of the tongue and/or face. The onset of extrapyramidal symptoms is a risk factor for tardive dyskinesia. If signs and symptoms of tardive dyskinesia appear, the discontinuation of all antipsychotics should be considered.
Neuroleptic malignant syndrome (NMS)
Neuroleptic Malignant Syndrome, characterised by hyperthermia, muscle rigidity, autonomic instability, altered consciousness and elevated serum creatine phosphokinase levels has been reported to occur with antipsychotics. Additional signs may include myoglobinuria (rhabdomyolysis) and acute renal failure. In this event, all antipsychotics, including RISPERDAL CONSTA, should be discontinued.
Parkinson’s disease and dementia with Lewy bodies
Physicians should weigh the risks versus the benefits when prescribing antipsychotics, including RISPERDAL CONSTA, to patients with Parkinson’s Disease or Dementia with Lewy Bodies (DLB). Parkinson’s Disease may worsen with risperidone. Both groups may be at increased risk of Neuroleptic Malignant Syndrome as well as having an increased sensitivity to antipsychotic medicinal products; these patients were excluded from clinical trials. Manifestation of this increased sensitivity can include confusion, obtundation, postural instability with frequent falls, in addition to extrapyramidal symptoms.
Hypersensitivity reactions
Although tolerability with oral risperidone should be established prior to initiating treatment with RISPERDAL CONSTA, rarely anaphylactic reactions have been reported during post-marketing experience in patients who have previously tolerated oral risperidone (see sections 4.2 and 4.8).
If hypersensitivity reactions occur, discontinue use of RISPERDAL CONSTA; initiate general supportive measures as clinically appropriate and monitor the patient until signs and symptoms resolve (see sections 4.3 and 4.8).
Hyperglycaemia and diabetes mellitus
Hyperglycaemia, diabetes mellitus, and exacerbation of pre-existing diabetes have been reported during treatment with RISPERDAL CONSTA. In some cases, a prior increase in body weight has been reported which may be a predisposing factor. Association with ketoacidosis has been reported very rarely and rarely with diabetic coma. Appropriate clinical monitoring is advisable in accordance with utilised antipsychotic guidelines. Patients treated with any atypical antipsychotic, including RISPERDAL CONSTA, should be monitored for symptoms of hyperglycaemia (such as polydipsia, polyuria, polyphagia and weakness) and patients with diabetes mellitus should be monitored regularly for worsening of glucose control.
Weight gain
Significant weight gain has been reported with RISPERDAL CONSTA use. Weight should be monitored regularly.
Hyperprolactinaemia
Hyperprolactinaemia is a common side effect of treatment with RISPERDAL CONSTA. Evaluation of the prolactin plasma level is recommended in patients with evidence of possible prolactin-related side effects (e.g. gynaecomastia, menstrual disorders, anovulation, fertility disorder, decreased libido, erectile dysfunction, galactorrhea).
Tissue culture studies suggest that cell growth in human breast tumours may be stimulated by prolactin. Although no clear association with the administration of antipsychotics has so far been demonstrated in clinical and epidemiological studies, caution is recommended in patients with relevant medical history. RISPERDAL CONSTA should be used with caution in patients with pre-existing hyperprolactinaemia and in patients with possible prolactin-dependent tumours.
QT prolongation
QT prolongation has very rarely been reported post-marketing. As with other antipsychotics, caution should be exercised when risperidone is prescribed in patients with known cardiovascular disease, family history of QT prolongation, bradycardia, or electrolyte disturbances (hypokalaemia, hypomagnesaemia), as it may increase the risk of arrhythmogenic effects, and in concomitant use with medicines known to prolong the QT interval.
Seizures
RISPERDAL CONSTA should be used cautiously in patients with a history of seizures or other conditions that potentially lower the seizure threshold.
Priapism
Priapism may occur with RISPERDAL CONSTA treatment due to its alpha-adrenergic blocking effects.
Body temperature regulation
Disruption of the body’s ability to reduce core body temperature has been attributed to antipsychotic medicines. Appropriate care is advised when prescribing RISPERDAL CONSTA to patients who will be experiencing conditions which may contribute to an elevation in core body temperature, e.g. exercising strenuously, exposure to extreme heat, receiving concomitant treatment with anticholinergic activity, or being subject to dehydration.
Venous thromboembolism
Cases of venous thromboembolism (VTE) have been reported with antipsychotic drugs. Since patients treated with antipsychotics often present with acquired risk factors for VTE, all possible risk factors for VTE should be identified before and during treatment with RISPERDAL CONSTA and preventative measures undertaken.
Intraoperative floppy iris syndrome
Intraoperative Floppy Iris Syndrome (IFIS) has been observed during cataract surgery in patients treated with medicines with alphala-adrenergic antagonist effect, including RISPERDAL CONSTA (see section 4.8).
IFIS may increase the risk of eye complications during and after the operation. Current or past use of medicines with alphala-adrenergic antagonist effect should be made known to the ophthalmic surgeon in advance of surgery. The potential benefit of stopping alpha 1-blocking therapy prior to cataract surgery has not been established and must be weighed against the risk of stopping the antipsychotic therapy.
Antiemetic effect
An antiemetic effect was observed in preclinical studies with risperidone. This effect, if it occurs in humans, may mask the signs and symptoms of overdosage with certain medicines or of conditions such as intestinal obstruction, Reye’s syndrome, and brain tumour.
Renal or hepatic impairment
Although oral risperidone has been studied, RISPERDAL CONSTA has not been studied in patients with renal or liver insufficiency. RISPERDAL CONSTA should be administered with caution in this group of patients (see section 4.2).
Administration
Care must be taken to avoid inadvertent injection of RISPERDAL CONSTA into a blood vessel.
Excipients
This medicinal product contains less than 1 mmol sodium (23 mg) per dose, i.e., essentially ‘sodium-free’.
4.5 Interaction with other medicinal products and other forms of interaction
The interactions of RISPERDAL CONSTA with co-administration of other drugs have not been systematically evaluated. The drug interaction data provided in this section are based on studies with oral RISPERDAL.
Pharmacodynamic-related interactions
Drugs known to prolong the QT interval
As with other antipsychotics, caution is advised when prescribing risperidone with medicinal products known to prolong the QT interval, such as antiarrhythmics (e.g. quinidine, dysopiramide, procainamide, propafenone, amiodarone, sotalol), tricyclic antidepressants (i.e., amitriptyline), tetracyclic antidepressant (i.e., maprotiline), some antihistamines, other antipsychotics, some antimalarials (i.e., quinine and mefloquine), and with medicines causing electrolyte imbalance (hypokalaemia, hypomagnesiaemia), bradycardia, or those which inhibit the hepatic metabolism of risperidone. This list is indicative and not exhaustive.
Centrally-acting drugs and alcohol
Risperidone should be used with caution in combination with other centrally-acting substances notably including alcohol, opiates, antihistamines and benzodiazepines due to the increased risk of sedation.
Levodopa and dopamine agonists
RISPERDAL CONSTA may antagonise the effect of levodopa and other dopamine agonists. If this combination is deemed necessary, particularly in end-stage Parkinson’s disease, the lowest effective dose of each treatment should be prescribed.
Drugs with hypotensive effect
Clinically significant hypotension has been observed post-marketing with concomitant use of risperidone and antihypertensive treatment.
Pharmacokinetic-related interactions
Risperidone is mainly metabolised through CYP2D6, and to a lesser extent through CYP3A4. Both risperidone and its active metabolite 9-hydroxyrisperidone are substrates of P-glycoprotein (P-gp). Substances that modify CYP2D6 activity, or substances strongly inhibiting or inducing CYP3A4 and/or P-gp activity, may influence the pharmacokinetics of the risperidone active antipsychotic fraction.
Strong CYP2D6 inhibitors
Co-administration of RISPERDAL CONSTA with a strong CYP2D6 inhibitor may increase the plasma concentrations of risperidone, but less so of the active antipsychotic fraction. Higher doses of a strong CYP2D6 inhibitor may elevate concentrations of the risperidone active antipsychotic fraction (e.g. paroxetine, see below). It is expected that other CYP2D6 inhibitors, such as quinidine, may affect the plasma concentrations of risperidone in a similar way. When concomitant paroxetine, quinidine, or another strong CYP2D6 inhibitor, especially at higher doses, is initiated or discontinued, the physician should re-evaluate the dosing of RISPERDAL CONSTA.
CYP3A4 and/or P-gp inhibitors
Co-administration of RISPERDAL CONSTA with a strong CYP3A4 and/or P-gp inhibitor may substantially elevate plasma concentrations of the risperidone active antipsychotic fraction. When concomitant itraconazole or another strong CYP3A4 and/or P-gp inhibitor is initiated or discontinued, the physician should re-evaluate the dosing of RISPERDAL
CONSTA.
CYP3A4 and/or P-gp inducers
Co-administration of RISPERDAL CONSTA with a strong CYP3A4 and/or P-gp inducer may decrease the plasma concentrations of the risperidone active antipsychotic fraction. When concomitant carbamazepine or another strong CYP3A4 and/or P-gp inducer is initiated or discontinued, the physician should re-evaluate the dosing of RISPERDAL CONSTA. CYP3A4 inducers exert their effect in a time-dependent manner, and may take at least 2 weeks to reach maximal effect after introduction. Conversely, on discontinuation, CYP3A4 induction may take at least 2 weeks to decline.
Highly protein-bound drugs
When RISPERDAL CONSTA is taken together with highly protein-bound drugs, there is no clinically relevant displacement of either drug from the plasma proteins.
When using concomitant medication, the corresponding label should be consulted for information on the route of metabolism and the possible need to adjust dosage.
Paediatric population
Interaction studies have only been performed in adults. The relevance of the results from these studies in paediatric patients is unknown.
Examples
Examples of drugs that may potentially interact or that were shown not to interact with risperidone are listed below:
Effect of other medicinal products on the pharmacokinetics of risperidone
Antibacterials:
• Erythromycin, a moderate CYP3A4 inhibitor and P-gp inhibitor, does not change the pharmacokinetics of risperidone and the active antipsychotic fraction.
• Rifampicin, a strong CYP3A4 inducer and a P-gp inducer, decreased the plasma concentrations of the active antipsychotic fraction.
Anticholinesterases:
• Donepezil and galantamine, both CYP2D6 and CYP3A4 substrates, do not show a clinically relevant effect on the pharmacokinetics of risperidone and the active antipsychotic fraction.
Antiepileptics:
• Carbamazepine, a strong CYP3A4 inducer and a P-gp inducer, has been shown to decrease the plasma concentrations of the active antipsychotic fraction of risperidone. Similar effects may be observed with e.g. phenytoin and phenobarbital which also induce CYP3A4 hepatic enzyme, as well as P-glycoprotein.
• Topiramate modestly reduced the bioavailability of risperidone, but not that of the active antipsychotic fraction. Therefore, this interaction is unlikely to be of clinical significance.
Antifungals:
• Itraconazole, a strong CYP3A4 inhibitor and a P-gp inhibitor, at a dosage of
200 mg/day increased the plasma concentrations of the active antipsychotic fraction by about 70%, at risperidone doses of 2 to 8 mg/day.
• Ketoconazole, a strong CYP3A4 inhibitor and a P-gp inhibitor, at a dosage of 200 mg/day increased the plasma concentrations of risperidone and decreased the plasma concentrations of 9-hydroxy-risperidone.
Antipsychotics:
• Phenothiazines may increase the plasma concentrations of risperidone but not those of the active antipsychotic fraction.
Antivirals:
• Protease inhibitors: No formal study data are available; however, since ritonavir is a strong CYP3A4 inhibitor and a weak CYP2D6 inhibitor, ritonavir and ritonavir-boosted protease inhibitors potentially raise concentrations of the risperidone active antipsychotic fraction.
Beta-blockers:
• Some beta-blockers may increase the plasma concentrations of risperidone but not those of the active antipsychotic fraction.
Calcium channel blockers:
• Verapamil, a moderate inhibitor of CYP3A4 and an inhibitor of P-gp, increases the plasma concentration of risperidone and the active antipsychotic fraction.
Gastrointestinal drugs:
• H2-receptor antagonists: Cimetidine and ranitidine, both weak inhibitors of CYP2D6 and CYP3A4, increased the bioavailability of risperidone, but only marginally that of the active antipsychotic fraction.
SSRIs and tricyclic antidepressants:
• Fluoxetine, a strong CYP2D6 inhibitor, increases the plasma concentration of risperidone, but less so of the active antipsychotic fraction.
• Paroxetine, a strong CYP2D6 inhibitor, increases the plasma concentrations of risperidone, but, at dosages up to 20 mg/day, less so of the active antipsychotic fraction. However, higher doses of paroxetine may elevate concentrations of the risperidone active antipsychotic fraction.
• Tricyclic antidepressants may increase the plasma concentrations of risperidone but not those of the active antipsychotic fraction. Amitriptyline does not affect the pharmacokinetics of risperidone or the active antipsychotic fraction.
• Sertraline, a weak inhibitor of CYP2D6, and fluvoxamine, a weak inhibitor of CYP3A4, at dosages up to 100 mg/day are not associated with clinically significant changes in concentrations of the risperidone active antipsychotic fraction. However, doses higher than 100 mg/day of sertraline or fluvoxamine may elevate concentrations of the risperidone active antipsychotic fraction.
Effect of risperidone on the pharmacokinetics of other medicinal products
Antiepileptics:
• Risperidone does not show a clinically relevant effect on the pharmacokinetics of valproate or topiramate.
Antipsychotics:
• Aripiprazole, a CYP2D6 and CYP3A4 substrate: Risperidone tablets or injections did not affect the pharmacokinetics of the sum of aripiprazole and its active metabolite, dehydroaripiprazole.
Digitalis glycosides:
• Risperidone does not show a clinically relevant effect on the pharmacokinetics of digoxin.
Lithium:
• Risperidone does not show a clinically relevant effect on the pharmacokinetics of lithium.
Concomitant use of risperidone with furosemide
• See section 4.4 regarding increased mortality in elderly patients with dementia concomitantly receiving furosemide.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no adequate data from the use of risperidone in pregnant women. Risperidone was not teratogenic in animal studies but other types of reproductive toxicity were seen (see section 5.3). The potential risk for humans is unknown.
Neonates exposed to antipsychotics (including RISPERDAL CONSTA) during the third trimester of pregnancy are at risk of adverse reactions including extrapyramidal and/or withdrawal symptoms that may vary in severity and duration following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, or feeding disorder. Consequently, newborns should be monitored carefully.
RISPERDAL CONSTA should not be used during pregnancy unless clearly necessary.
Breast-feeding
In animal studies, risperidone and 9-hydroxy-risperidone are excreted in the milk. It has been demonstrated that risperidone and 9-hydroxy-risperidone are also excreted in human breast milk in small quantities. There are no data available on adverse effects in breast-feeding infants. Therefore, the advantage of breast-feeding should be weighed against the potential risks for the child.
Fertility
As with other drugs that antagonise dopamine D2 receptors, RISPERDAL CONSTA elevates prolactin level. Hyperprolactinemia may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotropin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients.
There were no relevant effects observed in the non-clinical studies.
4.7 Effects on ability to drive and use machines
RISPERDAL CONSTA has minor or moderate influence on the ability to drive and use machines due to potential nervous system and visual effects (see section 4.8). Therefore, patients should be advised not to drive or operate machinery until their individual susceptibility is known.
4.8 Undesirable effects
The most frequently reported adverse drug reactions (ADRs) (incidence > 1/10) are: insomnia, anxiety, headache, upper respiratory tract infection, parkinsonism, and depression.
The ADRs that appeared to be dose-related included parkinsonism and akathisia.
Serious injection site reactions including injection site necrosis, abscess, cellulitis, ulcer, haematoma, cyst, and nodule were reported post-marketing. The frequency is considered not known (cannot be estimated from the available data). Isolated cases required surgical intervention.
The following are all the ADRs that were reported in clinical trials and post-marketing experience with risperidone by frequency category estimated from RISPERDAL CONSTA clinical trials. The following terms and frequencies are applied: very common (> 1/10), common (> 1/100 to < 1/10), uncommon (> 1/1,000 to < 1/100), rare (> 1/10,000 to < 1/1,000), and very rare (< 1/10,000).
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness.
|
System Organ Class |
Adverse Drug Reaction | ||||
|
Frequency | |||||
|
Very common |
Common |
Uncommon |
Rare |
Very Rare | |
|
Infections and infestations |
upper respiratory tract infection |
pneumonia, bronchitis, sinusitis, urinary tract infection, influenza |
respiratory tract infection, cystitis, ear infection, eye infection, tonsillitis, onychomycosis, cellulitis, infection, localised infection, viral infection, acarodermatitis, subcutaneous abscess | ||
|
Blood and lymphatic system disorders |
anaemia |
white blood cell count decreased, thrombocytopenia, haematocrit decreased |
agranulocytosis0, neutropenia, eosinophil count increased | ||
|
Immune system |
hypersensitivity |
anaphylactic | |||
|
disorders |
reaction0 | ||||
|
Endocrine disorders |
hyperprolactinaemia3 |
glucose urine present |
inappropriate antidiuretic hormone secretion | ||
|
Metabolism and nutrition disorders |
hyperglycaemia, weight increased, increased appetite, weight decreased, decreased appetite |
diabetes mellitusb, anorexia, blood triglycerides increased, blood cholesterol increased |
water intoxicationc, hypoglycemia, hyperinsulinaemiac, polydipsia |
diabetic ketoacidosis | |
|
Psychiatric disorders |
■ d insomnia , depression, anxiety |
sleep disorder, agitation, libido decreased |
mania, confusional state, anorgasmia, nervousness, nightmare |
blunted affect | |
|
Nervous system disorders |
parkinsonismd, headache |
sedation/somnolence, akathisiad, dystoniad, dizziness, dyskinesiad, tremor |
tardive dyskinesia, cerebral ischaemia, loss of consciousness, convulsiond, syncope, psychomotor hyperactivity, balance disorder, coordination abnormal, dizziness postural, disturbance in attention, dysarthria, dysgeusia, hypoaesthesia, paraesthesia |
neuroleptic malignant syndrome, cerebrovascular disorder, unresponsive to stimuli, depressed level of consciousness, diabetic coma, head titubation | |
|
Eye disorders |
vision blurred |
conjunctivitis, dry eye, lacrimation increased, ocular hyperaemia |
retinal artery occlusion, glaucoma, eye movement disorder, eye rolling, photophobia, eyelid margin crusting, floppy iris syndrome (intraoperative)c | ||
|
Ear and labyrinth disorders |
vertigo, tinnitus, ear pain | ||||
|
Cardiac disorders |
tachycardia |
atrial fibrillation, atrioventricular block, conduction disorder, electrocardiogram QT prolonged, bradycardia, electrocardiogram abnormal, palpitations |
sinus arrhythmia | ||
|
Vascular disorders |
hypotension, hypertension |
orthostatic hypotension |
pulmonary embolism, venous thrombosis, flushing | ||
|
Respiratory, thoracic and mediastinal disorders |
dyspnoea, pharyngolaryngeal pain, cough, nasal congestion |
hyperventilation, respiratory tract congestion, wheezing, epistaxis |
sleep apnoea syndrome, pneumonia aspiration, pulmonary congestion, rales, dysphonia, respiratory disorder |
|
Gastrointestinal disorders |
abdominal pain, abdominal discomfort, vomiting, nausea, constipation, gastroenteritis, diarrhoea, dyspepsia, dry mouth, toothache |
faecal incontinence, dysphagia, flatulence |
pancreatitis, intestinal obstruction, swollen tongue, faecaloma, cheilitis |
ileus | |
|
Skin and subcutaneous tissue disorders |
rash |
pruritus, alopecia, eczema, dry skin, erythema, skin discolouration, acne, seborrhoeic dermatitis |
drug eruption, urticaria, hyperkeratosis, dandruff, skin disorder, skin lesion |
angioedema | |
|
Musculoskeletal and connective tissue disorders |
muscle spasms, musculoskeletal pain, back pain, arthralgia |
blood creatine phosphokinase increased, joint stiffness, joint swelling, muscular weakness, neck pain |
rhabdomyolysis, posture abnormal | ||
|
Renal and urinary disorders |
urinary incontinence |
pollakiuria, urinary retention, dysuria | |||
|
Pregnancy, puerperium, and neonatal conditions |
drug withdrawal syndrome neonatalc | ||||
|
Reproductive system and breast disorders |
erectile dysfunction, amenorrhoea, galactorrhoea |
ejaculation disorder, menstruation delayed, menstrual disorderd, gynaecomastia, sexual dysfunction, breast pain, breast discomfort, vaginal discharge |
priapismc, breast engorgement, breast enlargement, breast discharge | ||
|
General disorders and administration site conditions |
oedemad, pyrexia, chest pain, asthenia, fatigue, pain, injection site reaction |
face oedema, chills, body temperature increased, gait abnormal, thirst, chest discomfort, malaise, feeling abnormal, indurationc |
hypothermia, body temperature decreased, peripheral coldness, drug withdrawal syndrome, discomfort | ||
|
Hepatobiliary disorders |
transaminases increased, gamma-glutamyltransferas increased |
hepatic enzyme increased |
jaundice | ||
|
Injury, poisoning and procedural complications |
fall |
procedural pain |
Hyperprolactinemia can in some cases lead to gynaecomastia, menstrual disturbances, amenorrhoea, anovulation, galactorrhea, fertility disorder, decreased libido, erectile dysfunction.
In placebo-controlled trials diabetes mellitus was reported in 0.18% in risperidone-treated subjects compared to a rate of 0.11% in placebo group. Overall incidence from all clinical trials was 0.43% in all risperidone-treated subjects.
c
Not observed in RISPERDAL CONSTA clinical studies but observed in post-marketing environment with risperidone.
Extrapyramidal disorder may occur: Parkinsonism (salivary hypersecretion, musculoskeletal stiffness, parkinsonism, drooling, cogwheel rigidity, bradykinesia, hypokinesia, masked facies, muscle tightness, akinesia, nuchal rigidity, muscle rigidity, parkinsonian gait, and glabellar reflex abnormal, parkinsonian rest tremor), akathisia (akathisia, restlessness, hyperkinesia, and restless leg syndrome), tremor, dyskinesia (dyskinesia, muscle twitching, choreoathetosis, athetosis, and myoclonus), dystonia. Dystonia includes dystonia, hypertonia, torticollis, muscle contractions involuntary, muscle contracture, blepharospasm, oculogyration, tongue paralysis, facial spasm, laryngospasm, myotonia, opisthotonus, oropharyngeal spasm, pleurothotonus, tongue spasm, and trismus. It should be noted that a broader spectrum of symptoms are included, that do not necessarily have an extrapyramidal origin. Insomnia includes initial insomnia, middle insomnia. Convulsion includes grand mal convulsion. Menstrual disorder includes menstruation irregular, oligomenorrhoea. Oedema includes generalised oedema, oedema peripheral, pitting oedema.
Undesirable effects noted with paliperidone formulations
Paliperidone is the active metabolite of risperidone, therefore, the adverse reaction profiles of these compounds (including both the oral and injectable formulations) are relevant to one another. In addition to the above adverse reactions, the following adverse reaction has been noted with the use of paliperidone products and can be expected to occur with RISPERDAL
CONSTA.
Cardiac disorders
Postural orthostatic tachycardia syndrome
Anaphylactic reaction
Rarely, cases of anaphylactic reaction after injection with RISPERDAL CONSTA have been reported during post-marketing experience in patients who have previously tolerated oral risperidone (see section 4.4).
Class effects
As with other antipsychotics, very rare cases of QT prolongation have been reported post-marketing with risperidone. Other class-related cardiac effects reported with antipsychotics which prolong QT interval include ventricular arrhythmia, ventricular fibrillation, ventricular tachycardia, sudden death, cardiac arrest and Torsades de Pointes.
Venous thromboembolism
Cases of venous thromboembolism, including cases of pulmonary embolism and cases of deep vein thrombosis, have been reported with antipsychotic drugs (frequency unknown).
Weight gain
In the 12-week double-blind, placebo-controlled trial, 9% of patients treated with RISPERDAL CONSTA, compared with 6% of patients treated with placebo, experienced a weight gain of > 7% of body weight at endpoint. In the 1-year, open-label study of RISPERDAL CONSTA, changes in body weight in individual patients were generally within ± 7% from baseline; 25% of patients had an increase in body weight of > 7%.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov .uk/yellowcard.
4.9 Overdose
While overdose is less likely to occur with parenteral than with oral medicinal products, information pertaining to oral is presented.
Symptoms
In general, reported signs and symptoms have been those resulting from an exaggeration of the known pharmacological effects of risperidone. These include drowsiness and sedation, tachycardia and hypotension, and extrapyramidal symptoms. In overdose, QT-prolongation and convulsions have been reported. Torsade de Pointes has been reported in association with combined overdose of oral RISPERDAL and paroxetine.
In case of acute overdose, the possibility of multiple drug involvement should be considered.
Treatment
Establish and maintain a clear airway and ensure adequate oxygenation and ventilation. Cardiovascular monitoring should commence immediately and should include continuous electrocardiographic monitoring to detect possible arrhythmias.
There is no specific antidote to RISPERDAL. Therefore appropriate supportive measures should be instituted. Hypotension and circulatory collapse should be treated with appropriate measures such as intravenous fluids and/or sympathomimetic agents. In case of severe extrapyramidal symptoms, anticholinergic medicinal product should be administered. Close medical supervision and monitoring should continue until the patient recovers.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Other antipsychotics, ATC code: N05AX08
Mechanism of action
Risperidone is a selective monoaminergic antagonist with unique properties. It has a high affinity for serotoninergic 5-HT2 and dopaminergic D2 receptors. Risperidone binds also to alpha1-adrenergic receptors, and, with lower affinity, to H1-histaminergic and alpha2-adrenergic receptors. Risperidone has no affinity for cholinergic receptors. Although risperidone is a potent D2 antagonist, that is considered to improve the positive symptoms of schizophrenia, it causes less depression of motor activity and induction of catalepsy than classical antipsychotics. Balanced central serotonin and dopamine antagonism may reduce extrapyramidal side effect liability and extend the therapeutic activity to the negative and affective symptoms of schizophrenia.
Clinical efficacy
The effectiveness of RISPERDAL CONSTA (25 mg and 50 mg) in the management of the manifestations of psychotic disorders (schizophrenia/schizoaffective disorder) was established in one 12-week, placebo-controlled trial in adult psychotic inpatients and outpatients who met the DSM-IV criteria for schizophrenia.
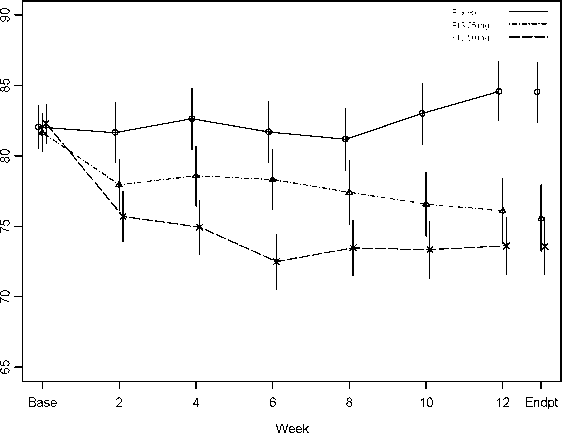
In a 12-week comparative trial in stable patients with schizophrenia, RISPERDAL CONSTA was shown to be as effective as the oral tablet formulation. The long-term (50 weeks) safety and efficacy of RISPERDAL CONSTA was also evaluated in an open-label trial of stable psychotic inpatients and outpatients who met the DSM-IV criteria for schizophrenia or schizoaffective disorder. Over time efficacy was maintained with RISPERDAL CONSTA (Figure 1).
Figure 1. Mean in total PANSS score over time (LOCF) in patients with schizophrenia.
5.2 Pharmacokinetic properties
Absorption
The absorption of risperidone from RISPERDAL CONSTA is complete.
After a single intramuscular injection with RISPERDAL CONSTA, the release profile consists of a small initial release of risperidone (< 1% of the dose), followed by a lag time of 3 weeks. The main release of risperidone starts from week 3 onwards, is maintained from 4 to 6 weeks, and subsides by week 7. Oral antipsychotic supplementation should therefore be given during the first 3 weeks of RISPERDAL CONSTA treatment (see section 4.2).
The combination of the release profile and the dosage regimen (intramuscular injection every two weeks) results in sustained therapeutic plasma concentrations. Therapeutic plasma concentrations remain until 4 to 6 weeks after the last RISPERDAL CONSTA injection.
After repeated intramuscular injections with 25 or 50 mg RISPERDAL CONSTA every two weeks, median trough and peak plasma concentrations of the active antipsychotic fraction fluctuated between 9.9-19.2 ng/ml and 17.9-45.5 ng/ml respectively. No accumulation of risperidone was observed during long term use (12 months) in patients who were injected with 25-50 mg every two weeks.
The above studies were conducted with gluteal intramuscular injection. Deltoid and gluteal intramuscular injections at the same doses are bioequivalent and, therefore, interchangeable.
Distribution
Risperidone is rapidly distributed. The volume of distribution is 1-2 l/kg. In plasma, risperidone is bound to albumin and alphal-acid glycoprotein. The plasma protein binding of risperidone is 90%; that of the active metabolite 9-hydroxy-risperidone is 77%.
Biotransformation and elimination
Risperidone is metabolised by CYP2D6 to 9-hydroxy-risperidone, which has a similar pharmacological activity as risperidone. Risperidone plus 9-hydroxy-risperidone form the active antipsychotic fraction. CYP2D6 is subject to genetic polymorphism. Extensive CYP2D6 metabolisers convert risperidone rapidly into 9-hydroxy-risperidone, whereas poor CYP2D6 metabolisers convert it much more slowly. Although extensive metabolisers have lower risperidone and higher 9-hydroxy-risperidone concentrations than poor metabolisers, the pharmacokinetics of risperidone and 9-hydroxy-risperidone combined (i.e., the active antipsychotic fraction), after single and multiple doses, are similar in extensive and poor metabolisers of CYP2D6.
Another metabolic pathway of risperidone is N-dealkylation. In vitro studies in human liver microsomes showed that risperidone at clinically relevant concentration does not substantially inhibit the metabolism of medicines metabolised by cytochrome P450 isozymes, including CYP1A2, CYP2A6, CYP2C8/9/10, CYP2D6, CYP2E1, CYP3A4, and CYP3A5. One week after oral risperidone administration, 70% of the dose is excreted in the urine and 14% in the faeces. In urine, risperidone plus 9-hydroxy-risperidone represent 35-45% of the orally administered dose. The remainder is inactive metabolites. The elimination phase is complete approximately 7 to 8 weeks after the last RISPERDAL CONSTA injection.
Linearity
The pharmacokinetics of risperidone are linear in the dose range of 25-50 mg injected every 2 weeks.
Elderly, hepatic and renal impairment
A single-dose PK-study with oral risperidone showed on average a 43% higher active antipsychotic fraction plasma concentrations, a 38% longer half-life and a reduced clearance of the active antipsychotic fraction by 30% in the elderly.
In adults with moderate renal disease the clearance of the active moiety was ~48% of the clearance in young healthy adults (age range 25-35 years). In adults with severe renal disease the clearance of the active moiety was ~31% of the clearance in young healthy adults. The half-life of the active moiety was 16.7 h in young adults, 24.9 h in adults with moderate renal disease (or ~1.5 times as long as in young adults), and 28.8 h in those with severe renal disease (or ~1.7 times as long as in young adults). Risperidone plasma concentrations were normal in patients with liver insufficiency, but the mean free fraction of risperidone in plasma was increased by 37.1%.
The oral clearance and the elimination half-life of risperidone and of the active moiety in adults with moderate and severe liver impairment were not significantly different from those parameters in young healthy adults.
Pharmacokinetic/pharmacodynamic relationship
There was no relationship between the plasma concentrations of the active antipsychotic fraction and the change in total PANSS (Positive And Negative Syndrome Scale) and total ESRS (Extrapyramidal Symptom Rating Scale) scores across the assessment visits in any of the phase III trials where efficacy and safety was examined.
Gender, race and smoking habits
A population pharmacokinetic analysis revealed no apparent effect of gender, race or smoking habits on the pharmacokinetics of risperidone or the active antipsychotic fraction.
5.3 Preclinical safety data
Similar to the (sub)chronic toxicity studies with oral risperidone in rats and dogs, the major effects of treatment with RISPERDAL CONSTA (up to 12 months of intramuscular administration) were prolactin-mediated mammary gland stimulation, male and female genital tract changes, and central nervous system (CNS) effects, related to the pharmacodynamic activity of risperidone. In a toxicity study in juvenile rats treated with oral risperidone, increased pup mortality and a delay in physical development was observed. In a 40-week study with juvenile dogs treated with oral risperidone, sexual maturation was delayed. Based on AUC, long bone growth was not affected in dogs at 3.6- times the maximum human oral exposure in adolescents (1.5mg/day); while effects on long bones and sexual maturation were observed at 15 times the maximum human oral exposure in adolescents.
Risperidone was not teratogenic in rat and rabbit. In rat reproduction studies with risperidone, adverse effects were seen on mating behaviour of the parents, and on birth weight and survival of the offspring. In rats, intrauterine exposure to risperidone was associated with cognitive deficits in adulthood. Other dopamine antagonists, when administered to pregnant animals, have caused negative effects on learning and motor development in the offspring.
RISPERDAL CONSTA administration to male and female rats for 12 and 24 months produced osteodystrophy at a dose of 40mg/kg/2 weeks. The effect dose for osteodystrophy in rats was on a mg/m2 basis 8 times the maximum recommended human dose and is associated with a plasma exposure 2 times the maximum anticipated exposure in humans at the maximum recommended dose. No osteodystrophy was observed in dogs treated for 12 months with RISPERDAL CONSTA up to 20mg/kg/2 weeks. This dose yielded plasma exposures up to 14 times the maximum recommended human dose.
There was no evidence of genotoxic potential.
As expected for a potent dopamine D2-antagonist, in oral carcinogenicity studies of risperidone in rats and mice, increases in pituitary gland adenomas (mouse), endocrine pancreas adenomas (rat), and mammary gland adenomas (both species) were seen.
In an intramuscular carcinogenicity study with RISPERDAL CONSTA in Wistar (Hannover) rats (doses of 5 and 40mg/kg/2 weeks), increased incidences of endocrine pancreas, pituitary gland, and adrenal medullary tumours were observed at 40mg/kg, while mammary gland tumours were present at 5 and 40mg/kg. These tumours observed upon oral and intramuscular dosing can be related to prolonged dopamine D2 antagonism and hyperprolactinaemia. Tissue culture studies suggest that cell growth in human breast tumours may be stimulated by prolactin. Hypercalcemia, postulated to contribute to an increased incidence of adrenal medullary tumours in RISPERDAL CONSTA-treated rats, was observed in both dose groups. There is no evidence to suggest that hypercalcemia might cause phaeochromocytomas in humans.
Renal tubular adenomas occurred in male rats treated with RISPERDAL CONSTA at 40mg/kg/2 weeks. No renal tumours occurred in the low dose, the NaCl 0.9%, or the microspheres vehicle control group. The mechanism underlying the renal tumours in RISPERDAL CONSTA-treated male Wistar (Hannover) rats is unknown. A treatment-related increase in renal tumour incidence did not occur in the oral carcinogenicity studies with Wistar (Wiga) rats or in Swiss mice administered oral risperidone. Studies conducted to explore the substrain differences in the tumour organ profile suggest that the Wistar (Hannover) substrain employed in the carcinogenicity study differs substantially from the Wistar (Wiga) substrain employed in the oral carcinogenicity study with respect to spontaneous age-related non-neoplastic renal changes, serum prolactin increases, and renal changes in response to risperidone. There are no data suggesting kidney-related changes in dogs treated chronically with RISPERDAL CONSTA.
The relevance of the osteodystrophy, the prolactin-mediated tumours and of the presumed rat substrain-specific renal tumours in terms of human risk is unknown.
Local irritation at the injection site in dogs and rats was observed after administration of high doses of RISPERDAL CONSTA. In a 24-month intramuscular carcinogenicity study in rats, no increased incidence of injection site tumours was seen in either the vehicle or active groups.
In vitro and in vivo, animal models show that at high doses risperidone may cause QT interval prolongation, which has been associated with a theoretically increased risk of torsade de pointes in patients.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Microspheres
[poly-(d,l-lactide-co-glycolide)]
Solvent Polysorbate 20 Carmellose sodium
Disodium hydrogen phosphate dihydrate
Citric acid anhydrous
Sodium chloride
Sodium hydroxide
Water for injection
6.2 Incompatibilities
This medicinal product must not be mixed with other medicinal products except those mentioned in section 6.6.
6.3 Shelf life
3 years at 2-8°C.
After reconstitution: Chemical and physical in-use stability has been demonstrated for 24 hours at 25°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 6 hours at 25C, unless reconstitution has taken place in controlled and validated aseptic conditions.
6.4 Special precautions for storage
The entire dose pack should be stored in the refrigerator (2-8°C).
If refrigeration is unavailable, RISPERDAL CONSTA can be stored at temperatures not exceeding 25°C for no more than 7 days prior to administration.
Store in the original package.
For storage conditions of the reconstituted medicinal product, see section 6.3.
6.5 Nature and contents of container
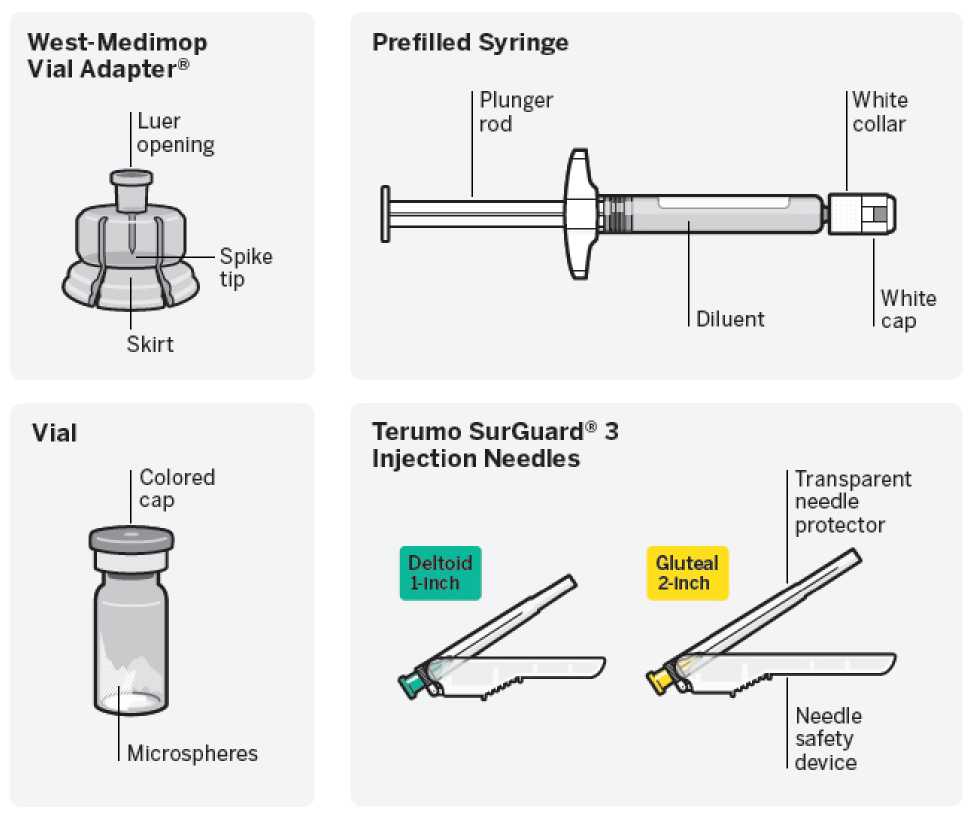
Needle-Free Vial Access Device
• One vial containing powder for prolonged-release suspension for injection
• One West-Medimop Vial Adapter® for reconstitution (referred as Vial Adapter)
• One prefilled syringe containing the solvent for RISPERDAL CONSTA
• Two Terumo SurGuard®3 needles for intramuscular injection (a 21G UTW 1-inch (0.8 mm x 25 mm) safety needle with needle protection device for deltoid administration and a 20G TW 2-inch (0.9 mm x 51 mm) safety needle with needle protection device for gluteal administration).
Risperdal Consta is available in packs containing 1 or 5 (bundled) packs. Not all pack sizes may be marketed.
6.6 Special precautions for disposal and other handling Important information
RISPERDAL® CONSTA® requires close attention to these step-by-step Instructions for Use to help ensure successful administration.
Use components provided
The components in this dose pack are specifically designed for use with RISPERDAL® CONSTA®. RISPERDAL® CONSTA® must be reconstituted only in the diluent supplied in the dose pack.
Do not substitute ANY components of the dose pack.
Do not store suspension after reconstitution
Administer dose as soon as possible after reconstitution to avoid settling.
Proper dosing
The entire contents of the vial must be administered to ensure intended dose of RISPERDAL® CONSTA® is delivered.
SINGLE-USE DEVICE
Do not reuse. Medical devices require specific material characteristics to perform as intended. These characteristics have been verified for single use only. Any attempt to re-process the device for subsequent re-use may adversely affect the integrity of the device or lead to deterioration in performance.
Dose pack contents
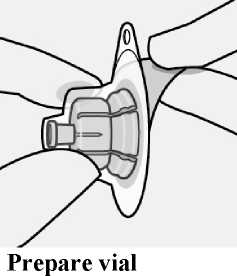
Take out dose pack Connect vial adapter to vial
Connect vial adapter to vial
adapter
Remove dose pack from the refrigerator and allow to sit at room temperature for at least 30 minutes before reconstituting.
Do not warm any other way.
Remove cap from vial
Flip off coloured cap from vial.
Wipe top of the grey stopper with an alcohol swab.
Allow to air dry.
Hold sterile blister as shown.
Peel back and remove paper backing.
Do not remove vial adapter from blister.
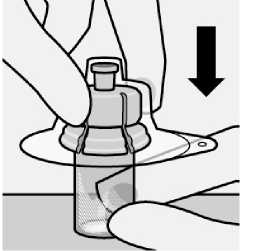
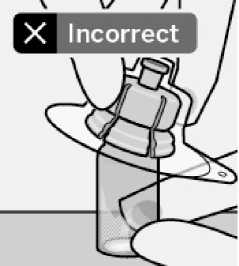
Place vial on a hard surface and hold by the base. Centre vial adapter over the grey rubber stopper. Push vial adapter straight down onto vial top until it snaps securely into place.
Do not remove grey rubber stopper.
Do not touch spike tip at any time. This will result in contamination.
Do not place vial adapter on at an angle or diluent may leak upon transfer to the vial.
Connect prefilled syringe to vial adapter
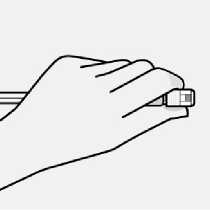
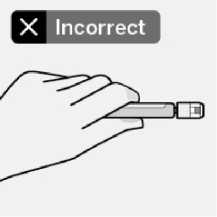
Use proper grip
Keep vial vertical to prevent leakage.
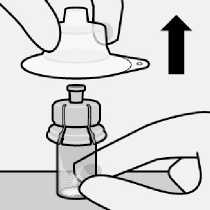
Hold base of vial and pull up on the sterile blister to remove.
The broken-off cap can be discarded.
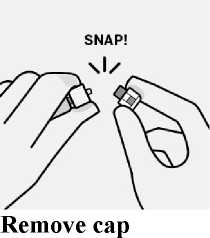
Remove sterile blister
-
Remove vial adapter from sterile blister only when you are ready to remove the white cap from the prefilled syringe.
Do not shake.
Do not touch exposed luer opening on vial adapter.
This will result in contamination.
Hold by white collar at the tip of the syringe.
Do not hold syringe by the glass barrel during assembly.
Holding the white collar, snap off the white cap.
Do not twist or cut off the white cap.
Do not touch syringe tip. This will result in contamination.
Connect syringe to vial adapter
Hold vial adapter by skirt to keep stationary.
Hold syringe by white collar then insert tip into the luer opening of the vial adapter.
Do not hold the glass syringe barrel. This may cause the white collar to loosen or detach.
Attach the syringe to the vial adapter with a firm clockwise twisting motion
until it feels snug.
Do not over-tighten. Over-tightening may cause the syringe tip to break.
Step 2
Reconstitute microspheres
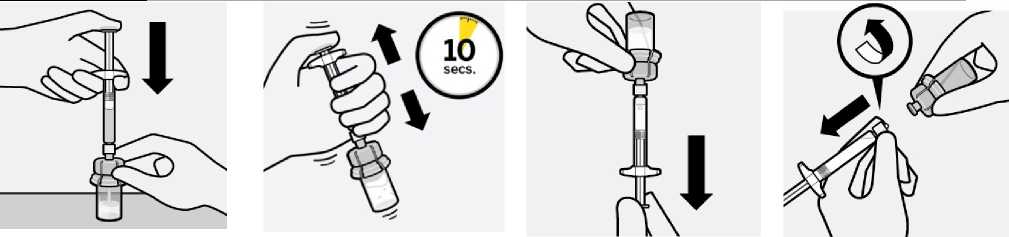
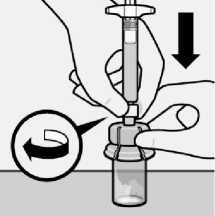
Inject diluent
Inject entire amount of diluent from syringe into the vial.
fA---
Vial contents will now be under pressure.
Keep holding the plunger rod down with thumb.
Transfer suspension to syringe
Invert vial completely. Slowly pull plunger rod down to withdraw entire contents from the vial into the syringe.
Suspend microspheres in diluent
Continuing to hold down the plunger rod, shake vigorously for at least 10 seconds, as
shown.
Check the suspension. When properly mixed, the suspension appears uniform, thick and milky in colour. Microspheres will be visible in the liquid.
Immediately proceed to the next step so suspension does not settle.
Remove vial adapter
Hold white collar on the syringe and unscrew from vial adapter.
Tear section of the vial label at the perforation. Apply detached label to the syringe for identification purposes.
Discard both vial and vial adapter appropriately.
Step 3
Attach needle
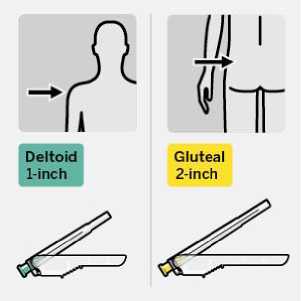
Select appropriate needle
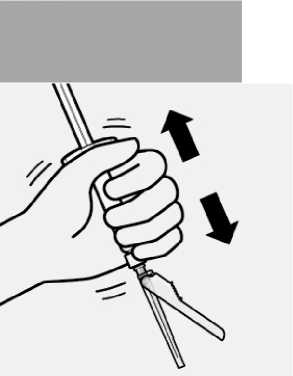
Choose needle based on injection location (gluteal or deltoid).
Attach needle
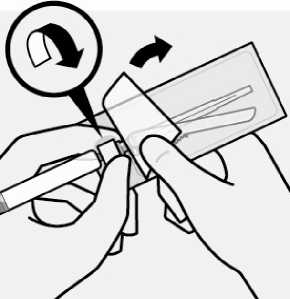
Peel blister pouch open part way and use to grasp the base of the needle, as shown.
Holding the white collar on the syringe, attach syringe to needle luer connection with a firm clockwise twisting motion until snug.
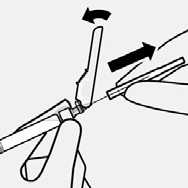
Resuspend microspheres
Fully remove the blister pouch.
Just before injection, shake syringe vigorously again, as some settling will have occurred.
Do not touch needle luer opening. This will result in contamination.
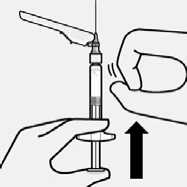
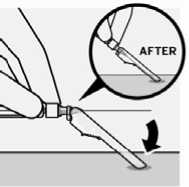
Remove air bubbles
Hold syringe upright and tap gently to make any air bubbles rise to the top. Slowly and carefully press plunger rod upward to remove air.
Remove transparent needle protector
Move the needle safety device back towards the syringe,
as shown. Then hold white collar on syringe and carefully pull the transparent needle protector straight off.
Do not twist transparent needle protector, as the luer connection may loosen.
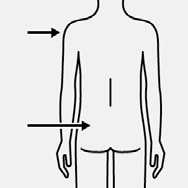
Inject
Immediately inject entire contents of syringe
intramuscularly (IM) into the gluteal or deltoid muscle of the patient.
Gluteal injection should be made into the upper-outer quadrant of the gluteal area.
Do not
administer
intravenously.
Secure needle in safety device
Using one hand. place needle safety device at a 45 degree angle on a hard, flat surface. Press down with a firm, quick motion until needle is fully engaged in safety device.
Avoid needle stick injury:
Do not use two hands.
Do not
intentionally disengage or mishandle the needle safety device.
Do not attempt to straighten the needle or engage the safety device if the needle is bent or damaged.
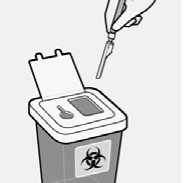
Properly dispose of needles
Check to confirm needle safety device is fully engaged.
Discard in an approved sharps container.
Also discard the unused needle provided in the dose pack.
MARKETING AUTHORISATION HOLDER
7
Janssen-Cilag Ltd
50-100 Holmers Farm Way
High Wycombe
Bucks
HP12 4EG
UK
8 MARKETING AUTHORISATION NUMBER(S)
PL 00242/0376
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE
AUTHORISATION
08/08/2002 / 28/02/2004
10 DATE OF REVISION OF THE TEXT
13/11/2015