Risperdal Consta 37.5Mg
PACKAGE LEAFLET: INFORMATION FOR THE USER 2053/2054/2055
24.02.16[25]
Risperdal Consta™ 25 mg Risperdal Consta™ 37.5 mg Risperdal Consta™ 50 mg
(risperidone)
The name of your medicine is Risperdal Consta 25mg/ Risperdal Consta 37.5mg/ Risperdal Consta 50mg but it will be referred to as Risperdal Consta throughout this leaflet.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section
4.
What is in this leaflet
1. What Risperdal Consta is and what it is used for
2. What you need to know before you use Risperdal Consta
3. How to use Risperdal Consta
4. Possible side effects
5. How to store Risperdal Consta
6. Contents of the pack and other information
1. What Risperdal Consta is and what it is used for
Risperdal Consta belongs to a group of medicines called ‘anti-psychotics'.
Risperdal Consta is used to maintain the treatment of schizophrenia, where you may see, hear or feel things that are not there, believe things that are not true or feel unusually suspicious, or confused.
Risperdal Consta is intended for patients who are currently treated with oral (e.g. tablets, capsules) antipsychotics.
Risperdal Consta can help alleviate the symptoms of your disease and stop your symptoms from coming back.
2. What you need to know before you use Risperdal Consta Do not use Risperdal Consta
• If you are allergic to risperidone or any of the other ingredients of this medicine (listed in Section 6).
Warnings and precautions
• If you have never taken any form of Risperdal, you should begin with oral Risperdal before beginning treatment with Risperdal Consta.
Talk to your doctor or pharmacist before using Risperdal Consta if:
• You have a heart problem. Examples include an irregular heart rhythm or if you are prone to low blood pressure or if you are using medicines for your blood pressure. Risperdal Consta may cause low blood pressure. Your dose may need to be adjusted.
• You know of any factors which would favour you having a stroke, such as high blood pressure, cardiovascular disorder or circulation disorders of the brain
• You have ever experienced involuntary movements of the tongue, mouth and face
• You have ever had a condition whose symptoms include high temperature, muscle stiffness, sweating or a lowered level of consciousness (also known as Neuroleptic Malignant Syndrome)
• You have Parkinson's disease or dementia
• You know that you have had low levels of white blood cells in the past (which may or may not have been caused by other medicines)
• You are diabetic
• You have epilepsy
• You are a man and have ever had a prolonged or painful erection
• You have difficulty controlling body temperature or overheating
• You have kidney problems
• You have liver problems
• You have an abnormally high level of the hormone prolactin in your blood or if you have a possible prolactin-dependent tumour
• You or someone else in your family has a history of blood clots, as medicines like these have been associated with formation of blood clots.
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using Risperdal or Risperdal Consta.
As dangerously low numbers of a certain type of white blood cell needed to fight infection in your blood has been seen very rarely with patients using Risperdal Consta, your doctor may check your white blood cell counts.
Even if you have previously tolerated oral risperidone, rarely allergic reactions occur after receiving injections of Risperdal Consta. Seek medical attention right away if you experience a rash, swelling of your throat, itching, or problems breathing as these may be signs of a serious allergic reaction.
Risperdal Consta may cause you to gain weight. Significant weight gain may adversely affect your health. Your doctor should regularly measure your body weight.
As diabetes mellitus or worsening of pre-existing diabetes mellitus have been seen with patients taking Risperdal, your doctor should check for signs of high blood sugar. In patients with pre-existing diabetes mellitus blood glucose should be monitored regularly.
Risperdal Consta commonly raises levels of a hormone called "prolactin". This may cause side effects such as menstrual disorders or fertility problems in women, breast swelling in men (see Possible side effects). If such side effects occur, evaluation of the prolactin level in the blood is recommended.
During an operation on the eye for cloudiness of the lens (cataract), the pupil (the black circle in the middle of your eye) may not increase in size as needed. Also, the iris (the coloured part of the eye) may become floppy during surgery and that may lead to eye damage. If you are planning to have an operation on your eye, make sure you tell your eye doctor that you are using this medicine.
Elderly people with dementia
Risperdal Consta is not for use in elderly people with dementia.
Medical treatment should be sought straight away if you or your carer notice a sudden change in your mental state or sudden weakness or numbness of your face, arms or legs, especially on one side, or slurred speech, even for a short period of time. These may be signs of a stroke.
People with kidney or liver problems
Although oral risperidone has been studied, Risperdal Consta has not been studied in patients with kidney or liver problems. Risperdal Consta should be administered with caution in this patient group.
Other medicines and Risperdal Consta
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
It is especially important to talk to your doctor or pharmacist if you are taking any of the following
• Medicines that work on your brain to help you calm down (benzodiazepines), or some medicines for pain (opiates), medicines for allergy (some antihistamines), as risperidone may increase the sedative effect of all of these.
• Medicines that may change the electrical activity of your heart, such as medicines for malaria, heart rhythm problems, allergies (anti-histamines), some antidepressants or other medicines for mental problems
• Medicines that cause a slow heart beat
• Medicines that cause low blood potassium (such as certain diuretics)
• Medicines for Parkinson's disease (such as levodopa)
• Medicines to treat raised blood pressure. Risperdal Consta can lower blood pressure
• Water tablets (diuretics) used for heart problems or swelling of parts of your body due to a build up of too much fluid (such as furosemide or chlorothiazide). Risperdal Consta taken by itself or with furosemide, may have an increased risk of stroke or death in elderly people with dementia.
The following medicines may reduce the effect of risperidone
• Rifampicin (a medicine for treating some infections)
• Carbamazepine, phenytoin (medicines for epilepsy)
• Phenobarbital
If you start or stop taking such medicines you may need a different dose of risperidone.
The following medicines may increase the effect of risperidone
• Quinidine (used for certain types of heart disease)
• Antidepressants such as paroxetine, fluoxetine, tricyclic antidepressants
• Medicines known as beta blockers (used to treat high blood pressure)
• Phenothiazines (such as medicines used to treat psychosis or to calm down)
• Cimetidine, ranitidine (blockers of the acidity of stomach)
• Itraconazole and ketoconazole (medicines for treating fungal infections)
• Certain medicines used in the treatment of HIV/AIDS, such as ritonavir
• Verapamil, a medicine used to treat high blood pressure and/or abnormal heart rhythm.
• Sertraline and fluvoxamine, medicines used to treat depression and other psychiatric disorders.
If you start or stop taking such medicines you may need a different dose of risperidone.
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using Risperdal Consta.
Risperdal Consta with food, drink and alcohol
You should avoid drinking alcohol when using Risperdal Consta.
Pregnancy, breast-feeding and fertility
• If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. Your doctor will decide if you can use it
• The following symptoms may occur in newborn babies, of mothers that have used Risperdal Consta in the last trimester (last three months of their pregnancy): shaking, muscle stiffness, and/or weakness, sleepiness, agitation, breathing problems, and difficulty in feeding. If your baby develops any of these symptoms you may need to contact your doctor.
• Risperdal Consta can raise your levels of a hormone called "prolactin" that may impact fertility (see Possible side effects).
Driving and using machines
Dizziness, tiredness, and vision problems may occur during treatment with Risperdal Consta. Do not drive or use any tools or machines without talking to your doctor first.
Risperdal Consta contains less than 1 mmol sodium (23 mg) per dose,
i.e., essentially ‘sodium-free'.
3. How to use Risperdal Consta
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Risperdal Consta is given as an intramuscular injection either in the arm or buttock every two weeks, administered by a health care professional. Injections should be alternated between the right and left sides, and should not be given intravenously.
The recommended dose is as follows:
Adults
Starting dose
If your daily dose of oral (e.g. tablets) risperidone was 4 mg or less for the last two weeks, your starting dose should be 25 mg Risperdal Consta.
If your daily dose of oral (e.g. tablets) risperidone was more than 4 mg for the last two weeks, you may be given 37.5 mg Risperdal Consta as a starting dose.
If you are currently treated with other oral antipsychotics than risperidone, your starting dose of Risperdal Consta will depend on your current treatment. Your doctor will choose Risperdal Consta 25 mg or 37.5 mg.
Your doctor will decide on the dose of Risperdal Consta that is right for you.
Maintenance dose
• The usual dose is 25 mg every two weeks as an injection
• A higher dose of 37.5 or 50 mg may be necessary. Your doctor will decide on the dose of Risperdal Consta that is right for you
• Your doctor may prescribe oral Risperdal for the first three weeks following your first injection.
If you are given more Risperdal Consta than you should
• People who have been given more Risperdal Consta than they should have experienced the following symptoms: sleepiness, tiredness, abnormal body movements, problems with standing and walking, dizziness from low blood pressure, and abnormal heart beats. Cases of abnormal electrical conduction in the heart and convulsion have been reported
• See a doctor right away.
If you stop using Risperdal Consta
You will lose the effects of the medicine. You should not stop this medicine unless told to do so by your doctor as your symptoms may return. Be sure not to miss your appointments when you are supposed to receive your injections every two weeks. If you cannot keep your appointment, be sure to contact your doctor right away to discuss another date when you can come in for your injection. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Use in children and adolescents
Risperdal Consta is not for people who are under 18 years old.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately if you:
• Experience blood clots in the veins, especially in the legs (symptoms include swelling, pain, and redness in the leg), which may travel through blood vessels to the lungs causing chest pain and difficulty breathing. If you notice any of these symptoms seek medical advice immediately
• Have dementia and experience a sudden change in your mental state or sudden weakness or numbness of your face, arms or legs, especially on one side, or slurred speech, even for a short period of time. These may be signs of a stroke
• Experience fever, muscle stiffness, sweating or a lowered level of consciousness (a disorder called “Neuroleptic Malignant Syndrome”). Immediate medical treatment may be needed
• Are a man and experience prolonged or painful erection. This is called priapism. Immediate medical treatment may be needed
• Experience involuntary rhythmic movements of the tongue, mouth and face. Withdrawal of risperidone may be needed
• Experience severe allergic reaction characterised by fever, swollen mouth, face, lip or tongue, shortness of breath, itching, skin rash or drop in blood pressure. Even if you have previously tolerated oral risperidone, rarely allergic reactions occur after receiving injections of Risperdal Consta.
The following side effects may happen:
Very common side effects (may affect more than 1 in 10 people)
• Common cold symptoms
• Difficulty falling or staying asleep
• Depression, anxiety
• Parkinsonism: This condition may include: slow or impaired movement, sensation of stiffness or tightness of the muscles (making your movements jerky), and sometimes even a sensation of movement "freezing up" and then restarting. Other signs of parkinsonism include a slow shuffling walk, a tremor while at rest, increased saliva and/ or drooling, and a loss of expression on the face.
• Headache.
Common side effects (may affect up to 1 in 10 people)
• Pneumonia, infection of the chest (bronchitis), sinus infection
• Urinary tract infection, feeling like you have the flu, anemia
• Raised levels of a hormone called "prolactin" found in a blood test (which may or may not cause symptoms). Symptoms of high prolactin occur uncommonly and may include in men breast swelling, difficulty in getting or maintaining erections, decreased sexual desire or other sexual dysfunction. In women they may include breast discomfort, leakage of milk from the breasts, missed menstrual periods, or other problems with your cycle or fertility problems.
• High blood sugar, weight gain, increased appetite, weight loss, decreased appetite
• Sleep disorder, irritability, decreased sexual drive, restlessness, feeling sleepy, or less alert
• Dystonia. This is a condition involving slow or sustained involuntary contraction of muscles. While it can involve any part of the body (and may result in abnormal posture), dystonia often involves muscles of the face, including abnormal movements of the eyes, mouth, tongue or jaw.
• Dizziness
• Dyskinesia: This is a condition involving involuntary muscle movements, and can include repetitive, spastic or writhing movements, or twitching.
• Tremor (shaking)
• Blurry vision
• Rapid heart rate
• Low blood pressure, chest pain, high blood pressure
• Shortness of breath, sore throat, cough, stuffy nose
• Abdominal pain, abdominal discomfort, vomiting, nausea, stomach or intestinal infection, constipation, diarrhoea, indigestion, dry mouth, toothache
• Rash
• Muscle spasms, bone or muscle ache, back pain, joint pain
• Incontinence (lack of control) of urine
• Erectile dysfunction
• Loss of menstrual periods
• Leakage of milk from the breasts
• Swelling of the body, arms or legs, fever, weakness, fatigue (tiredness),
• Pain
• A reaction at the injection site, including itching, pain or swelling
• Increased liver transaminases in your blood, increased GGT (a liver enzyme called gamma-glutamyltransferase) in your blood
• Fall.
Uncommon side effects (may affect up to 1 in 100 people)
• Infection of the breathing passages, bladder infection, ear infection
• eye infection, tonsillitis, fungal infection of the nails, infection of the skin, an infection confined to a single area of skin or part of the body, viral infection, skin inflammation caused by mites, abscess under the skin
• White blood cell count decreased, decrease in platelets (blood cells that help you stop bleeding), decrease in red blood cells
• Allergic reaction
• Sugar in the urine, diabetes or worsening of diabetes
• Loss of appetite resulting in malnutrition and low body weight
• High blood triglycerides (a fat), increased cholesterol in your blood
• Elated mood (mania), confusion, Inability to reach orgasm, nervousness, nightmares
• Tardive dyskinesia (twitching or jerking movements that you cannot control in your face, tongue, or other parts of your body). Tell your doctor immediately if you experience involuntary rhythmic movements of the tongue, mouth and face. Withdrawal of Risperdal Consta may be needed
• Sudden loss of blood supply to brain (stroke or "mini" stroke)
• Loss of consciousness, convulsion (fits), fainting
• A restless urge to move parts of your body, balance disorder, abnormal coordination, dizziness upon standing, disturbance in attention, problems with speech, loss or abnormal sense of taste, reduced sensation of skin to pain and touch, a sensation of tingling, pricking, or numbness of skin
• Eye infection or "pink eye", dry eye, increased tears, redness of the eyes
Risperdal Consta 25mg Risperdal Consta 37.5mg Risperdal Consta 50mg
- PL 30900/2053
- PL 30900/2054
- PL 30900/2055
POM
Risperdal Consta requires close attention to the step-by-step "Instructions for Use" to help ensure successful administration and help avoid difficulties in the use of the kit.
4.
6.
14 . RESUSPENSION OF RISPERDAL CONSTA WILL BE
NECESSARY PRIOR TO ADMINISTRATION AS SETTLING WILL OCCUR OVER TIME ONCE PRODUCT IS RECONSTITUTED. RESUSPEND THE MICROSPHERES IN THE SYRINGE BY SHAKING VIGOROUSLY.
• Sensation of spinning (vertigo), ringing in the ears, ear pain
• Atrial fibrillation (an abnormal heart rhythm), an interruption in conduction between the upper and lower parts of the heart, abnormal electrical conduction of the heart, prolongation of the QT interval from your heart, slow heart rate, abnormal electrical tracing of the heart (electrocardiogram or ECG), a fluttering or pounding feeling in your chest (palpitations)
• Low blood pressure upon standing (consequently, some people taking Risperdal Consta may feel faint, dizzy, or may pass out when they stand up or sit up suddenly)
• Fast, shallow breathing, congestion of breathing passages, wheezing, Nosebleeds
• Stool incontinence, difficulty swallowing, excessive passing of gas or wind
• Itching, hair loss, eczema, dry skin, skin redness, skin discoloration, acne, flaky, itchy scalp or skin
• An increase of CPK (creatine phosphokinase) in your blood, an enzyme which is sometimes released with muscle breakdown
• Joint stiffness, joint swelling, muscle weakness, neck pain
• Frequent passing of urine, inability to pass urine, pain when passing urine
• Ejaculation disorder, a delay in menstrual periods, Missed menstrual periods or other problems with your cycle (females), Development of breasts in men, Sexual dysfunction, Breast pain, Breast discomfort, Vaginal discharge
• Swelling of the face, mouth, eyes, or lips
• Chills, an increase in body temperature
• A change in the way you walk
• Feeling thirsty, feeling unwell, chest discomfort, feeling "out of sorts"
• Hardening of the skin
• Increased liver enzymes in your blood
• Procedural pain.
Rare side effects (may affect up to 1 in 1,000 people)
• Decrease in the type of white blood cells that help to protect you against infection
• Inappropriate secretion of a hormone that controls urine volume
• Low blood sugar
• Excessive drinking of water
• Lack of emotion
• Neuroleptic malignant syndrome (confusion, reduced or loss of consciousness, high fever, and severe muscle stiffness)
• Low level of consciousness
• Shaking of the head
• Problems with movement of your eyes, eye rolling, oversensitivity of the eyes to light
• Eye problems during cataract surgery. During cataract surgery, a condition called intraoperative floppy iris syndrome (IFIS) can happen if you use or have used Risperdal Consta. If you need to have cataract surgery, be sure to tell your eye doctor if you use or have used this medicine.
• Irregular heart beat
• Dangerously low numbers of a certain type of white blood cell needed to fight infection in your blood, increase in eosinophils (a type of white blood cell) in your blood, blood clot in the legs, blood clot in the lungs
• Trouble breathing during sleep (sleep apnea)
• Pneumonia caused by inhaling food, lung congestion, crackly lung sounds, voice disorder breathing passage disorder
• Inflammation of the pancreas, a blockage in the bowels
• Very hard stool
• Rash on skin related to drug
• Hives (or "nettle rash"), thickening of skin, dandruff, skin disorder, skin lesion
• Breakdown of muscle fibers and pain in muscles (rhabdomyolysis)
• Abnormal posture
• Breast enlargement, discharge from the breasts
• Decreased body temperature, discomfort
• Yellowing of the skin and the eyes (jaundice).
• Severe allergic reaction characterised by fever, swollen mouth, face, lip or tongue, shortness of breath, itching, skin rash and sometimes drop in blood pressure
• Dangerously excessive intake of water
• Increased insulin (a hormone that controls blood sugar levels) in your blood
• Blood vessel problems in the brain
• Unresponsive to stimuli
• Coma due to uncontrolled diabetes
• Sudden loss of vision or blindness
• Glaucoma (increased pressure within the eyeball), eyelid margin crusting
• Flushing, swollen tongue
• Chapped lips
• Priapism (a prolonged penile erection that may require surgical treatment)
• Enlargement of the glands in your breasts
• A decrease in body temperature, coldness in arms and legs
• Symptoms of drug withdrawal.
Very rare side effects (may affect up to 1 in 10,000 people)
• Life-threatening complications of uncontrolled diabetes.
• Serious allergic reaction with swelling that may involve the throat and lead to difficulty breathing.
• Lack of bowel muscle movement that causes blockage
The following side effect has been seen with the use of another medicine called paliperidone that is very similar to risperidone, so these can also be expected with Risperdal Consta: Rapid heartbeat upon standing.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Risperdal Consta
Store the entire dose pack in the refrigerator (2-8°C). If refrigeration is unavailable, the pack can be stored at room temperature (below 25°C) for a maximum of 7 days before use.
Protect from light. Store in the original package.
Use within 6 hours of reconstitution (if stored at or below 25oC).
Keep out of the sight and reach of children.
Do not use Risperdal Consta after the expiry date which is stated on the carton. The expiry date refers to the last day of that month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information What Risperdal Consta contains
The active substance is risperidone.
Each Risperdal Consta powder and solvent for prolonged-release suspension for injection contains either 25 mg, 37.5 mg or 50 mg of risperidone.
The other ingredient in Risperdal Consta is Poly-(D,L-Lactide-Co-Glycolide) 7525 DL JN1.
Solvent contains: Citric acid anhydrous, disodium hydrogen phosphate dihydrate, polysorbate 20, carmellose sodium, sodium chloride, sodium hydroxide and water for injections.
What Risperdal Consta looks like and contents of the pack
The Risperdal Consta package contains everything required to reconstitute (make up) and administer the product:
• One vial containing Risperdal Consta extended release microspheres
• One Alaris SmartSite needle-free vial access device for reconstitution
• One prefilled syringe containing 2 ml solvent for Risperdal Consta
• Two needles for intramuscular injection (a 21G UTW 1 -inch (0.8 mm x 25 mm) safety needle with Needle-Pro safety device for deltoid administration and a 20G TW 2-inch (0.9mm x 50 mm) safety needle with Needle-Pro safety device for gluteal administration).
Product Licence Holder and Manufacturer
The manufacturer is Janssen Pharmaceutica NV, Turnhoutseweg 30, B-2340 Beerse, Belgium. Procured from within the EU by the Product Licence holder: Tenolol Ltd, 5 Sandridge Close, Harrow, Middlesex, HA1 1XD. Repackaged by Servipharm Ltd.
Leaflet revision and issue date 24.02.16[25]
Risperdal Consta is a trademark of Johnson & Johnson
IMPORTANT INFORMATION FOR HEALTHCARE PROFESSIONALS Instructions for Needle-Free Vial Access Device
Risperdal Consta extended release microspheres in the vial must be reconstituted only in the solvent in the syringe supplied in the dose pack, and must be administered with only the appropriate needle supplied in the dose pack for gluteal (2-inch (50 mm) needle) or deltoid (1 -inch (25 mm) needle) administration. Do not substitute any components in the dose pack. To assure that the intended dose of risperidone is delivered, the full contents from the vial must be administered. Administration of partial contents may not deliver the intended dose of risperidone. It is recommended to administer immediately after reconstitution.
Remove the dose pack of Risperdal Consta from the refrigerator and allow it to come to room temperature for approximately 30 minutes prior to reconstitution.
Contents of the dose pack:
• One small bottle containing the powder for prolonged-release suspension for injection (within this powder is the active substance, risperidone). One syringe filled with 2 ml of clear, colourless liquid to be
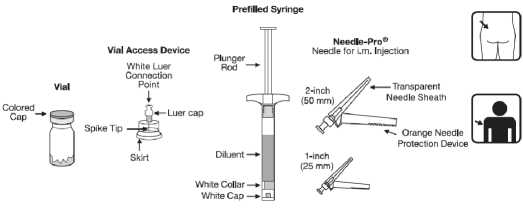
added to the powder for prolonged-release suspension for injection.
• One Alaris™ SmartSite® Needle-Free Vial Access Device for reconstitution
• Two needles for intramuscular injection (a 21G UTW 1 -inch (0.8 mm x 25 mm) safety needle with Needle-Pro safety device for deltoid administration and a 20G TW 2-inch (0.9 mm x 50 mm) safety needle with Needle-Pro safety device for gluteal administration).
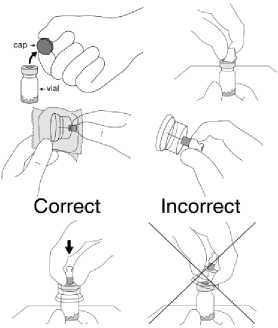
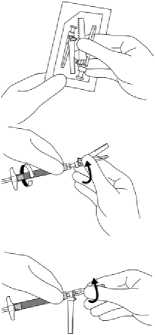
1. Flip off the plastic coloured cap from the vial. Do not remove the grey rubber stopper. Wipe the top of the grey rubber stopper with an alcohol wipe and allow to dry.
2. Peel back the blister pouch and remove the SmartSite Needle-Free Vial Access Device by holding between the white luer cap and the skirt.
Do not touch the spike tip of the access device at any time.
3. It is very important that the SmartSite Needle-Free Vial Access Device be placed on the vial correctly or the diluent could leak upon transfer to the vial.
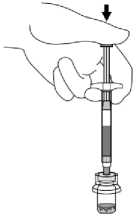
Place the vial on a hard surface. Hold the base of the vial. Orient the SmartSite Needle-Free Vial Access Device vertically over the vial so that the spike tip is at the center of the vial's rubber stopper.
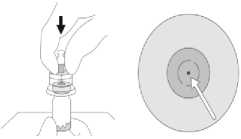
With a straight downward push press the spike tip of the SmartSite Needle-Free Vial Access Device through the centre of the vial's rubber stopper until the device securely snaps onto the vial top.
Hold the base of the vial and swab the syringe connection point (blue circle) of the SmartSite Needle-Free Vial Access Device with an alcohol wipe and allow to dry prior to attaching the syringe to the SmartSite Needle-Free Vial Access Device.
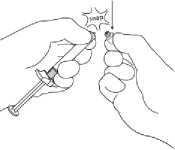
5. The prefilled syringe has a white tip consisting of 2 parts: a white collar and a smooth white cap. To open the syringe, hold the syringe by the white collar and snap off the smooth white cap (DO NOT TWIST OR CUT OFF THE WHITE CAP). Remove the white cap together with the
rubber tip cap inside.
For all syringe assembly steps, hold the syringe only by the white collar located at the tip of the syringe. Be careful not to overtighten components when assembling. Holding the white collar will help to prevent the white collar from getting detached and ensure a good connection to the syringe. Overtightening connections may cause syringe component parts to loosen from the syringe body.
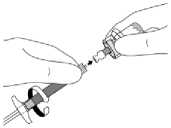
While holding the white collar of the syringe, insert and press the syringe tip into the blue circle of the SmartSite Needle-Free Vial Access Device and twist in a clockwise motion to secure the connection of the syringe to the SmartSite Needle-Free Vial Access Device (avoid over tightening).
Hold the skirt of the vial access device during attachment to prevent it from spinning.
Keep the syringe and the SmartSite Needle-Free Vial Access Device aligned.
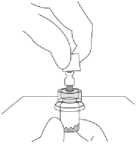
7. Inject the entire contents of the syringe containing the solvent into the vial.
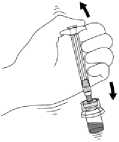
8. Shake the vial VIGOROUSLY while holding the plunger rod down with the thumb for a minimum of 10 seconds to ensure a homogeneous suspension. When properly mixed, the suspension appears uniform, thick, and milky in colour. The microspheres will be visible in liquid, but no dry microspheres remain.
DO NOT STORE THE VIAL AFTER RECONSTITUTION OR THE SUSPENSION MAY SETTLE.
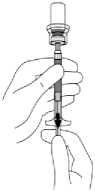
9. Invert the vial completely and SLOWLY withdraw the entire contents of the suspension from the vial into the syringe. Tear the section of the vial label at the perforation and apply the detached label to the syringe for identification purposes.
10. While holding the white collar of the syringe, unscrew the syringe from the SmartSite Needle-Free Vial Access Device.
Discard both the vial and vial access device appropriately.
11. Open the needle pack and select the appropriate needle provided with the kit. Do nOt touch the connection part of the needle, only touch the transparent sheath of the needle.
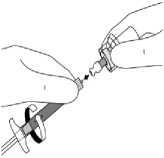
For GLUTEAL injection, select the 20G TW 2-inch (0.9 mm x 50 mm) needle (longer needle with yellow coloured hub).
For DELTOID injection, select the 21G UTW 1-inch (0.8 mm x 25 mm) needle (shorter needle with green coloured hub).
12. To prevent contamination, be careful not to touch the orange Needle-Pro safety device's luer connector. While holding the white collar of the syringe, attach the luer connection of the orange Needle-Pro safety device to the syringe with an easy clockwise twisting motion.
13. While continuing to hold the white collar of the syringe, grasp the transparent needle sheath and seat the needle firmly on the orange Needle-Pro safety device with a push and a clockwise twist. Seating the needle will help ensure a secure connection between the needle and the orange Needle-Pro safety device while conducting the following steps.
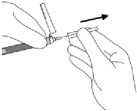
15. While holding the white collar of the syringe, pull the transparent needle sheath straight away from the needle. DO NOT TWIST the sheath as the luer connections may be loosened.
16. Tap the syringe gently to make any air bubbles rise to the top.
Remove air in syringe by depressing the plunger rod carefully and slowly, while holding the needle in an upright position. Inject the entire contents of the syringe intramuscularly into the selected gluteal or deltoid muscle of the patient immediately. Gluteal injection should be made into the upper-outer quadrant of the gluteal area.
DO NOT ADMINISTER INTRAVENOUSLY.
WARNING: To avoid a needle stick injury with a contaminated needle:
• Do not use free hand to press the Needle-Pro safety device over the needle.
• Do not intentionally disengage the Needle-Pro safety device
• Do not attempt to straighten the needle or engage Needle-Pro safety device if the needle is bent or damaged
• Do not mishandle the Needle-Pro safety device as it may cause the needle to protrude from the Needle-Pro safety device.
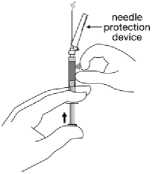
17. After injection is complete, press the needle into the orange Needle-Pro safety device using a one-handed technique. Perform a one-handed technique by GENTLY pressing the orange Needle-Pro safety device against a flat surface. AS THE ORANGE NEEDLE-PRO SAFETY DEVICE IS PRESSED, THE NEEDLE WILL FIRMLY ENGAGE INTO THE ORANGE NEEDLE-PRO SAFETY DEVICE.
Visually confirm that the needle is fully engaged into the orange Needle-Pro safety device before discarding. Discard needle appropriately. Also discard the other (unused) needle provided in the dose pack.
Do Not Reuse: Medical devices require specific material characteristics to perform as intended. These characteristics have been verified for single use only. Any attempt to reprocess the device for subsequent reuse may adversely affect the integrity of the device or lead to deterioration in performance.