Rivastigmine 2Mg/Ml Oral Solution
PACKAGE LEAFLET: INFORMATION FOR THE USER
Rivastigmine 2mg/ml oral solution
Read all of this leaflet carefully before you start taking this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Rivastigmine is and what it is used for
2. Before you take Rivastigmine
3. How to take Rivastigmine
4. Possible side effects
5. How to store Rivastigmine
6. Further information
1. WHAT RIVASTIGMINE IS AND WHAT IT IS USED FOR
The active substance of Rivastigmine is rivastigmine.
Rivastigmine belongs to a class of substances called cholinesterase inhibitors.
Rivastigmine is used to treat the symptoms of mild to moderately severe Alzheimer’s dementia. It is also used for the treatment of dementia in patients with Parkinson’s disease.
2. BEFORE YOU TAKE RIVASTIGMINE
Do NOT take Rivastigmine
- if you are allergic (hypersensitive) to rivastigmine (the active substance in Rivastigmine) or to any of the other ingredients of Rivastigmine listed in section 6 of this leaflet.
- if you have a skin reaction spreading beyond the patch size, if there is a more intense local reaction (such as blisters, increasing skin inflammation, swelling) and if it does not improve within 48 hours after removal of the transdermal patch.
If this applies to you, tell your doctor and do not take Rivastigmine.
Take special care with Rivastigmine
- if you have or have ever had, irregular heartbeat.
- if you have or have ever had, an active stomach ulcer.
- if you have or have ever had,, difficulties in passing urine.
- if you have or have ever had, seizures.
- if you have or have ever had, asthma or severe respiratory disease.
- if you have or have ever had, impaired kidney function.
- if you have or have ever had, impaired liver function.
- if you suffer from trembling.
- if you have a low body weight.
- if you have gastrointestinal reactions such as feeling sick (nausea), being sick (vomiting) and diarrhoea. You may become dehydrated (losing too much fluid) if vomiting or diarrhea are prolonged.
If any of these apply to you, your doctor may need to monitor you more closely while you are on this medicine.
If you have not taken Rivastigmine for several days, do not take the next dose until you have talked to your doctor.
The use of Rivastigmine in children and adolescents (age below 18 years) is not recommended. Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Rivastigmine should not be given at the same time as other medicines with similar effects to Rivastigmine. Rivastigmine might interfere with anticholinergic medicines (medicines used to relieve stomach cramps or spasms, to treat Parkinson’s disease or to prevent travel sickness).
If you have to undergo surgery whilst taking Rivastigmine tell your doctor before you are given any anaesthetics, because Rivastigmine may exaggerate the effects of some muscle relaxants during anaesthesia.
Pregnancy and breast-feeding
Tell your doctor if you become pregnant during treatment. It is preferable to avoid the use of Rivastigmine during pregnancy, unless clearly necessary.
You should not breast-feed during treatment with Rivastigmine.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Your doctor will tell you whether your illness allows you to drive vehicles and use machines safely. Rivastigmine may cause dizziness and somnolence, mainly at the start of treatment or when increasing the dose. If you feel dizzy or sleepy, do not drive, use machines or perform any tasks that require your attention.
Important information about some of the ingredients of Rivastigmine
Rivastigmine contains sucrose. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
One of the excipients in rivastigmine oral solution is sodium benzoate. Benzoic acid is a mild irritant to the skin, eyes and mucous membrane.
3. HOW TO TAKE RIVASTIGMINE
Always take Rivastigmine exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
How to start treatment
Your doctor will tell you what dose of Rivastigmine to take.
• Treatment usually starts with a low dose.
• Your doctor will slowly increase your dose depending on how you respond to the treatment.
• The highest dose that should be taken is 6.0 mg twice a day.
Your doctor will regularly check if the medicine is working for you. Your doctor will also monitor your weight whilst you are taking this medicine.
If you have not taken Rivastigmine for several days, do not take the next dose until you have talked to your doctor.
Taking this medicine
• Tell your caregiver that you are taking Rivastigmine.
• To benefit from your medicine, take it every day.
• Take Rivastigmine twice a day, in the morning and evening, with food.
How to use this medicine
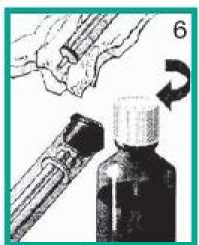
1. Preparing the bottle and syringe
• Take the syringe out of its protective case.
• Push down and turn the child resistant cap to open bottle.

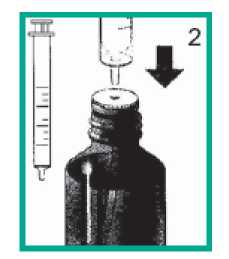
2 Attaching the syringe to the bottle
• Push the nozzle of the syringe into the hole in the white stopper.
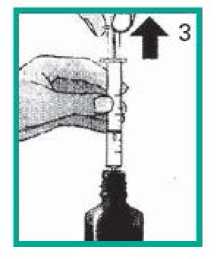
2. Filling the syringe
• Pull the plunger upwards until it reaches the right mark for the dose that your doctor has prescribed.
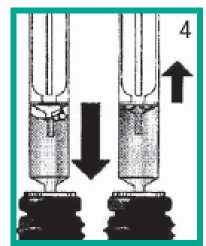
Removing bubbles
• Push down and pull plunger a few times to get rid of any large bubbles.
• A few tiny bubbles are not important and will not affect your dose in any way.
• Check the dose is still correct.
• Then, remove the syringe from the bottle.
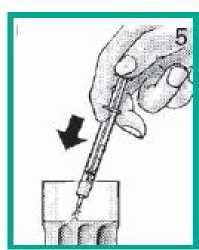
4. Taking your medicine
• Swallow your medicine straight from the syringe.
• You can also mix your medicine with water in a small glass. Stir and drink all of the mixture.
5. After using the syringe
• Wipe the outside of the syringe with a clean tissue.
• Then, put the syringe back in its protective case.
• Put the child resistant cap back on the bottle to close it.
If you take more Rivastigmine than you should
If you accidentally take more Rivastigmine that you should, inform your doctor. You may require medical attention. Some people who have accidentally taken too much Rivastigmine have experienced feeling sick (nausea), being sick (vomiting), diarrhoea, high blood pressure and hallucinations. Slow heart beat and fainting may also occur.
If you forget to take Rivastigmine
If you find you have forgotten to take your dose of Rivastigmine, wait and take the next dose at the usual time. Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Rivastigmine can cause side effects, although not everybody gets them.
You may tend have side effects more often when you start your medicine or when your dose is increased. Usually the side effects will slowly go away as your body gets used to the medicine.
The frequencies are defined as:
Very common (affects more than 1 patient in 10)
Common (affects 1 to 10 patients in 100)
Uncommon (affects 1 to 10 patients in 1000)
Rare (affects 1 to 10 patients in 10,000)
Very rare (affects less than 1 patient in 10,000)
Not known (frequency cannot be estimated from the available data)
Very common
• Feeling dizzy
• Loss of appetite
• Stomach problems such as feeling sick (nausea) or being sick (vomiting), diarrhoea
Common
• Anxiety
• Sweating
• Headache
• Heartburn
• Weight loss
• Stomach Pain
• Feeling agitated
• Feeling tired or weak
• Generally feeling unwell
• Trembling or feeling confused
Uncommon
• Depression
• Difficulty in sleeping
• Fainting or accidentally falling
• Changes in how well your liver is working
Rare
• Chest pain
• Rash, itching
• Fits (seizures)
• Ulcers in your stomach or intestine
Very rare
• High blood pressure
• Urinary tract infection
• Seeing things that are not there (hallucinations)
• Problems with your heartbeat such as fast or slow heartbeat
• Bleeding in the gut - shows as blood in stools or when being sick
• Inflammation of the pancreas - the signs include serious upper stomach pain, often with feeling sick (nausea) or being sick (vomiting)
• The signs of Parkinson’s disease get worse or getting similar signs - such as stiff muscles, difficulty in carrying out movements
Not known
• Being violently sick (vomiting) that can cause tearing of the tube that connects your mouth with your stomach (oesophagus).
• Dehydration (losing too much fluid)
• Liver disorders (yellow skin, yellowing of the whites of eyes, abnormal darkening of the urine or unexplained nausea, vomiting, tiredness and loss of appetite)
• Aggression, feeling restless
• Uneven heartbeat
Patients with dementia and Parkinson’s disease
These patients have some side effects more often. They also have some additional side effects:
Very common
• Trembling
• Fainting
• Accidentally falling
Common
• Anxiety
• Feeling restless,
• Slow and fast heartbeat
• Difficulty in sleeping
• Too much saliva and dehydration
• Unusually slow movements or movements you cannot control
• The signs of Parkinson’s disease get worse or gettting similar signs - such as stiff muscles, difficulty in carrying out movements and muscle weakness
Uncommon
• Uneven heartbeat and poor control of movements
Other side effects seen with Rivastigmine transdermal patches and which may occur with oral solution:
Common
• Fever
• Severe confusion
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. HOW TO STORE RIVASTIGMINE
Keep out of the reach and sight of children.
Do not use Rivastigmine after the expiry date which is stated on the bottle and the carton after EXP. The expiry date refers to the last day of that month.
Do not refrigerate or freeze.
Use Rivastigmine within 2 months after first opening of the bottle.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION What Rivastigmine contains
- The active substance is rivastigmine. Each ml contains rivastigmine tartrate corresponding to rivastigmine base 2.0 mg.
- The other ingredients are sodium benzoate (E211), calcium hydrogen phosphate, anhydrous, lemon flavour (containing sucrose), quinoline yellow (E104), phosphoric acid,concentrated and purified water.
What Rivastigmine looks like and contents of the pack
Rivastigmine Oral solution is supplied as a 120 ml of a clear yellow solution in an amber glass bottle with a child-resistant closure and dip tube. The oral solution is packaged with a 4 ml oral syringe graduated to 0.5 ml, 1.0 ml, 1.5 ml, 2.0 ml, 2.5 ml, 3.0 ml, 3.5 ml and 4.0 ml in a plastic tube container.
Marketing Authorisation Holder and Manufacturer
Marketing Authorsation Holder: TEVA UK Limited, Eastbourne, BN22 9AG, UK Manufacturer:
Teva Czech Industries s.r.o, Opava-Komarov, Czech Republic OR*
Teva Operations Poland Sp.z.o.o, ul Mogilska 80. 31-546, Krakow, Poland OR*
TEVA Pharmaceutical Works Private Limited Company, Pallagi ut 13, 4042 Debrecen, Hungary OR*
TEVA Pharmaceutical Works Private Limited Company, H-2100 Godollo, Tancsics Mihaly ut 82, Hungary
OR*
Teva UK Limited, Brampton Road, Hampden Park, Eastbourne, BN22 9AG This leaflet was last revised in July 2012.
PL 00289/1529 * Only the BRS used will be printed on the final leaflet