Rizatriptan 10Mg Orodispersible Tablets
Out of date information, search another|
h Hd Vi 1 |
Ref: |
231-30-89053-B LEA RIZATRIPTAN 10mg OD TAB TUK <DEB |
14 June 2013 | ||||
|
TEVA UK LIMITED |
Version: |
1 |
Trackwise Parent: N/A |
Child: |
N/A | ||
|
PL Number(s), MA Holder & Packer: |
PL 00289/1323. TEVA UK Limited Licence, Teva Regulatory Team. Packed at Debrecen. | ||||||
F. P. Code:
231-10-01356
231-10-01357
N/A
Added by 3rd Party N/A
Added by 3rd Party Univers
Dimensions:
Colours:
(PANTONE® is a registered trademark of Pantone, Inc.)
PANTONE® GREEN C
L:
W:
D:
Foil Width: Perforated:
460 mm 160 mm
N/A
N/A
N/A
BLACK
EAN Code:
Pharma Code:
Edge Code:
Third party code Fonts:
Base Font Size: 9 Pt
IMPORTANT: Artwork, text and content must not be reset, remade, amended or altered. The only exceptions to this are: bleeds, chokes, spreads or other print related adjustments required for reproduction by the supplier. We must receive a copy of any 3rd Party Supplier’s Proof before approval to print will be granted.
PACKAGE LEAFLET: INFORMATION FOR THE USER
Read all of this leaflet carefully before you
start taking this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
IN THIS LEAFLET:
1. What Rizatriptan 10 mg Orodispersible Tablets are and what they are used for
2. Before you take Rizatriptan 10 mg Orodispersible Tablets
3. How to take Rizatriptan 10 mg Orodispersible Tablets
4. Possible side effects
5. How to store Rizatriptan 10 mg Orodispersible Tablets
6. Further information
OWHAT RIZATRIPTAN 10 mg
ORODISPERSIBLE TABLETS ARE AND WHAT THEY ARE USED FOR
Rizatriptan belongs to a class of medicines called selective serotonin 5-HT1B/1D receptor agonists.
Your doctor has prescribed Rizatriptan to treat the headache phase of your migraine attack.
Treatment with Rizatriptan:
Reduces swelling of blood vessels surrounding the brain. This swelling results in the headache pain of a migraine attack.
BEFORE YOU TAKE RIZATRIPTAN 10 mg ORODISPERSIBLE TABLETS
Do NOT take Rizatriptan if
• you are allergic (hypersensitive) to rizatriptan benzoate or any of the other ingredients of this medicine • you have moderately severe or severe high blood pressure, or mild high blood pressure that is not controlled by medication
• you have or have ever had heart problems including heart attack or pain on the chest (angina) or you have experienced heart disease related signs • you have severe liver or severe kidney problems
• you have had a stroke (cerebrovascular accident CVA) or mini stroke (transient ischaemic attack TIA)
• you have blockage problems with your arteries (peripheral vascular disease)
• you are taking monoamine oxidase (MAO) inhibitors such as moclobemide, phenelzine, tranylcypromine, or pargyline (drugs against depression), or linezolid (an antibiotic), or if it has been less than two weeks since you stopped taking MAO inhibitors
• you are now taking ergotamine-type medications, such as ergotamine or dihydro-ergotamine to treat your migraine or methysergide to prevent a migraine attack
• you are taking any other medicine in the same class, such as sumatriptan, naratriptan or zolmitriptan to treat your migraine. (See Taking other medicines below).

fluoxetine or serotonin norepinephrine reuptake inhibitors (SNRIs) such as venlafaxine, and duloxetine for depression as this combination can cause a severe reaction (see section 4 for possible side effects).
• you have had short lived symptoms including chest or throat pain and tightness.
If you take Rizatriptan too often this may result in you getting a chronic headache. In such cases you should contact your doctor as you may have to stop taking Rizatriptan.
Please tell your doctor or pharmacist about your symptoms. Your doctor will decide if you have migraine. You should take Rizatriptan only for a migraine attack. Rizatriptan should not be used to treat headaches that might be caused by other, more serious conditions.
Taking other medicines
Do not take Rizatriptan:
• if you are already taking a 5HT1B/1D agonist (sometimes referred to as 'triptans'), such as sumatriptan, naratriptan or zolmitriptan
• if you are taking a monoamine oxidase (MAO) inhibitor such as moclobemide, phenelzine, tranylcypromine, linezolid, or pargyline or if it has been less than two weeks since you stopped taking an MAO inhibitor
• if you use ergotamine-type medicines such as ergotamine or dihydro-ergotamine to treat your migraine
• if you use methysergide to prevent a migraine attack.
The above listed medicines when taken with Rizatriptan may increase the risk of side effects.
You should wait at least 6 hours after taking Rizatriptan before you take ergotamine-type medicines such as ergotamine or dihydro-ergotamine or methysergide.
You should wait at least 24 hours after taking ergotamine-type medicines before taking Rizatriptan.
Ask your doctor for instructions and the risks about taking Rizatriptan
• if you are taking propranolol (see section 3 How to take Rizatriptan)
• if you are taking SSRIs such as sertraline, escitalopram oxalate, and fluoxetine or SNRIs such as venlafaxine, and duloxetine for depression.
Please tell your doctor or pharmacist if you are taking or have recently taken or plan to take, any other medicines including medicines obtained without a prescription. This includes herbal medicines and those you normally take for a migraine. This is because Rizatriptan can affect the way some medicines work. Also other medicines can affect Rizatriptan.
Taking Rizatriptan with food and drink
Rizatriptan can take longer to work if it is taken after food. Although it is better to take it on an empty stomach, you can still take it if you have eaten.
Pregnancy and breast-feeding
It is not known whether Rizatriptan is harmful to an unborn baby when taken by a pregnant woman.
Talk to your doctor before taking this medicine if you are pregnant, planning to get pregnant or are breast-feeding. Your doctor will decide whether you can take this medicine if you are pregnant. Breast-feeding should be avoided for 24 hours after treatment.
TJ
3"
CD
-i
3
CD
n
O
Q_
a>
If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before taking Rizatriptan.
0)
"O
o
u
ro
E
!—
ro
_C
o_
Take special care with Rizatriptan 10 mg
Orodispersible Tablets
Before you take Rizatriptan, tell your doctor
or pharmacist, if:
• you have any of the following risk factors for heart disease: high blood pressure, diabetes, you smoke or you are using nicotine substitution, your family has a history of heart disease, you are a man over 40 years of age, or you are a postmenopausal woman
• you have kidney or liver problems
• you have a particular problem with the way your heart beats (bundle branch block)
• your headache is associated with dizziness, difficulty in walking, lack of coordination or weakness in the leg and arm
• you use herbal preparation containing St. John's wort
• you have had allergic reaction like swelling of the face, lips, tongue and/or throat which may cause difficulty breathing and/or swallowing (angioedema)
• you are taking selective serotonin reuptake inhibitors (SSRIs) such as sertraline, escitalopram oxalate, and
Ask your doctor or pharmacist for advice before taking any medicine.
Use in children and adolescents
There is limited experience with the use of Rizatriptan in children and adolecents under 18 years of age, therefore children and adolecents should not be given Rizatriptan.
Use in patients older than 65 years
There have been no full studies to look at how safe and effective Rizatriptan is amongst patients older than 65 years.
Driving and using machines
You may feel sleepy or dizzy while taking Rizatriptan. If this happens, do not drive or use any tools or machines.
Important information about some of the ingredients of Rizatriptan
Phenylketonuric patients: Rizatriptan Orodispersible Tablets contain aspartame (E951) a source of phenylalanine. May be harmful for people with phenylketonuria.
This medicine contains lactose. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
|
TFWil |
Ref: |
231-30-89053-B LEA RIZATRIPTAN 10mg OD TAB TUK <DEB |
14 JUNE 2013 | ||||
|
TEVA UK LIMITED |
Version: |
1 |
Trackwise Parent: N/A |
Child: |
N/A | ||
|
PL Number(s), MA Holder & Packer: |
PL 00289/1323. TEVA UK Limited Licence, Teva Regulatory Team. Packed at Debrecen. | ||||||
F. P. Code:
231-10-01356
231-10-01357
N/A
Added by 3rd Party N/A
Added by 3rd Party Univers
Dimensions:
Colours:
(PANTONE® is a registered trademark of Pantone, Inc.)
PANTONE® GREEN C
L:
W:
D:
Foil Width: Perforated:
460 mm 160 mm
N/A
N/A
N/A
BLACK
EAN Code:
Pharma Code:
Edge Code:
Third party code Fonts:
Base Font Size: 9 Pt
IMPORTANT: Artwork, text and content must not be reset, remade, amended or altered. The only exceptions to this are: bleeds, chokes, spreads or other print related adjustments required for reproduction by the supplier. We must receive a copy of any 3rd Party Supplier’s Proof before approval to print will be granted.
©HOW TO TAKE RIZATRIPTAN 10 mg ORODISPERSIBLE TABLETS
Rizatriptan is used to treat migraine attacks. Take Rizatriptan as soon as possible after your migraine headache has started. Do not use it to prevent an attack.
Always take Rizatriptan exactly as your doctor has told you. You should check with your doctor or your pharmacist if you are not sure.
The usual dose is 10 mg.
You should leave at least 2 hours between taking propranolol and Rizatriptan up to a maximum of 2 doses in a 24-hour period.
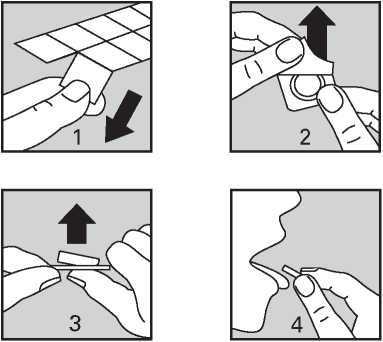
Rizatriptan is available as an orodispersible tablet that dissolves in the mouth.
Do not handle the tablets with wet hands as the tablets may break up.
1. Hold the blister strip at the edges and separate one blister cell from the rest of the strip by gently tearing along the perforations around it.
2. Carefully peel off the backing.
3. Gently push the tablet out.
4. Put the tablet in your mouth. It will
dissolve directly in your mouth, so that it can be easily swallowed.
• feeling sick (nausea), dry mouth, vomiting, diarrhoea
• feeling of heaviness in parts of the body
• pain in abdomen or chest.
Uncommon
• unsteadiness when walking (ataxia), dizziness (vertigo), blurred vision
• confusion, insomnia, nervousness
• high blood pressure (hypertension); thirst, indigestion (dyspepsia)
• itching and lumpy rash (hives)
• neck pain, feeling of tightness in parts of the body, stiffness, muscle weakness.
Rare
• bad taste in your mouth
• fainting (syncope), a syndrome called "serotonin syndrome" that may cause
TJ
3"
CD
-i
3
HI
n
O
Q.
rD
If migraine returns within 24 hours
In some patients, migraine symptoms can return within a 24-hour period. If your migraine does return you can take an additional dose of Rizatriptan. You should always wait at least 2 hours between doses.
If the first tablet does not provide any relief, do not take a second tablet.
If you do not get relief from the pain within 2 hours of taking a dose of Rizatriptan, you should not take a second dose to treat the same attack.
It is still likely, however, that you will respond to Rizatriptan during your next attack.
side effects like coma, unstable blood pressure, extremely high body temperature, lack of muscle coordination, agitation, and hallucinations
• facial pain, wheezing
• heart attack, spasm of blood vessels of the heart, stroke. They generally occur in patients with risk factors for heart and blood vessel disease (high blood pressure, diabetes, smoking, use of nicotine substitution, family history of heart disease or stroke, man over 40 years of age, postmenopausal women, particular problem with the way your heart beats (bundle branch block)).
Not known:
• seizure (convulsions/fits)
• spasm of blood vessels of the extremities including coldness and numbness of the hands or feet
• allergic reaction like swelling of the face, lips, tongue and/or throat which may cause difficulty breathing and/or swallowing (angioedema); rash, severe shedding of the skin including accompanied by fever (toxic epidermal necrolysis).
• irregular or slower heart beat, ECG abnormalities
• pain in the lower left side of the stomach and bloody diarrhoea (ischaemic colitis)
• muscle pain
Tell your doctor right away if you have symptoms of allergic reactions, serotonin syndrome, heart attack or stroke.
In addition, tell your doctor if you experience any symptoms that suggest an allergic reaction (such as a rash or itching) after taking Rizatriptan.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
HOW TO STORE RIZATRIPTAN 10 mg ORODISPERSIBLE TABLETS

Do not take more than 2 doses of Rizatriptan in a 24-hour period, (for example, do not take more than two 10 mg orodispersible tablets in a 24-hour period). You should always wait at least 2 hours between doses.
Keep out of the reach and sight of children.
Do not use Rizatriptan after the expiry date which is stated on the carton and the blister after EXP The expiry date refers to the last day of the month.
If your condition worsens, seek medical attention.
Store in original package in order to protect from moisture.
If you take more Rizatriptan than you should
If you take more Rizatriptan than you should, talk to your doctor or pharmacist straight away. Take the medicine pack with you.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Signs of overdose can include dizziness, drowsiness, vomiting, fainting and slow heart rate.
If you have further questions on the use of this product ask your doctor or pharmacist.

POSSIBLE SIDE EFFECTS
Like all medicines, Rizatriptan can cause side effects, although not everybody gets them. The following side effects may happen with this medicine.
In studies, the most common side effects reported were dizziness, sleepiness and tiredness.
The following side effects have been reported at the approximate frequencies shown:
_very common: affects more than 1 user in 10
at T5
common: affects 1 to 10 users in 100 uncommon: affects 1 to 10 users in 1,000 rare: affects 1 to 10 users in 10,000 very rare: affects less than 1 user in 10,000 not known: frequency cannot be estimated from the available data
TO -C Q.
Common
• tingling (paresthesia), headache, decreased sensitivity of skin (hypesthesia), decreased mental sharpness, tremor
• fast or irregular heart beat (palpitation), very fast heartbeat (tachycardia)
• flushing (redness of the face lasting a short time), hot flushes, sweating
• throat discomfort, difficulty breathing (dyspnea)
^ FURTHER INFORMATION
What Rizatriptan 10 mg Orodispersible Tablets contain
The active substance of the Rizatriptan Orodispersible Tablets is rizatriptan. One 10 mg orodispersible tablet contains 10 mg rizatriptan as 14.53 mg of rizatriptan benzoate.
The other ingredients of Rizatriptan Orodispersible Tablets are:
Lactose monohydrate, maize starch, mannitol (E421), pregelatinized starch (maize), aspartame (E951), peppermint flavour, Silica colloidal anhydrous, sodium stearyl fumarate.
What Rizatriptan 10 mg Orodispersible Tablets look like and contents of the pack
Rizatriptan 10 mg Orodispersible Tablets: are white to off white, round, flat orodispersible tablets with bevelled edges, embossed with 'IZ' on one side and '10' on the other side. Pack sizes: 2, 3, 6, 12, 18, 28 or 30 orodispersible tablets per pack.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
TEVA UK Limited, Eastbourne,
BN22 9AG, UK.
Manufacturer
Teva Pharmaceutical Works Private Limited Company, Pallagi ut 13, 4042 Debrecen, HUNGARY
This leaflet was last revised in 06/2013
PL 00289/1323
■inizD 89053-B
TEVA UK LIMITED 460 x 160