Rocuronium 10 Mg/Ml Solution For Injection / Infusion
Out of date information, search anotherPackage Insert MOxxxxx/xx GB
Size 180 x 588 mm
PACKAGE LEAFLET: INFORMATION FOR THE USER
Rocuronium 10 mg/ml solution for injection / infusion
Rocuronium Bromide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you..
* of this medicine is 'Ro et it will be referred to as 'Roc
What Is In this leaflet:
u are allergic to any muscle relaxant u have a kidney, a liver or a biliary disease ou have a heart disease or a disease ling your blood circulation
if you have a disease affecting the and muscles (neuromuscular disea
4. P
you ever developed a low body emperature during an anaethetic
1. WHAT ROCURONIUM IS AND WHAT IT IS USED FOR
e a low calcium le e a low potassium le
Th
ensive care unit f a tube into yc
high magnesium
f a tube int
from dehydration an increased amount of acids
e an increased amount of carbon
er from an excessive loss of weight
verweight or elderly
2. WHAT YOU NEED TO KNOW BEFORE YOU USE ROCURONIUM
Do not use Rocuronium
- if you are allergic to rocu bromide ion or any of the this medicine (listed in sec
Warnings and precautions
Talk to your doctor, pharmacist or nui
r medicines and Rocuronium antibiotics
anti-depressants: medicines used to treat de-■ression (eg. lithium salts mao inhibitors) edicines used for the treatment of heart diseases or high blood pressure (e.g.
- diuretics or water pills (medicines which increase the amount of urine)
- some laratives such as magnesium salts
- quinine (used to treat cramps and infections)
- medicines used for epilepsy treatment (e.g. phenytoin, carbamazepine)
- corticosteriods
- medicines used for the treatment of myasthenia gravis (neostigmine, pyridostigmine)
- azathioprine (used for transplant rejection prevention and treatment of auto-immune diseases)
- theophylline (used for the treatment of asthma)
- noradrenaline (a hormone which impacts blood pressure and other body functions)
- potassium chloride
- calcium chloride
- medicines used for the treatment or prevention of a virus infection (protease inhibitors: gabexate, ulinastatin)
Please note:
You may be given other medicines during the procedure which can influence the effects of rocuronium. These include certain anaesthetics (e.g. local anaesthetics, inhalational anaesthetics) and other muscle relaxants and protamines which reverse the anticoagulant effect (prevention of blood clots) of heparin. Your doctor will take this into account when he/she is dedding the correct dose of Rocuronium for you.
Pregnancy and breast-feeding If you are pregnant or breast-feeding , think you may be pregnant or are planning to have a taby, ask your doctor, pharmacist or nurse for advice before using this medicine.
For rocuronium bromide, no clinical data on exposed pregnancies and breast-feeding women are available. Rocuronium should only be given to pregnant and nursing women when the doctor decides that the benefits outweigh the risks. Rocuronium may be given
Driving and using machines
Rocuronium has a major influence on driving and
using machines.
Your doctor should advise you when you can start driving and using machines again. You should always be accompanied home by a responsible adult after your treatment.
Important information about some of the ingredients of Rocuronium
This medicinal product contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially ’sodium-free’.
3. HOW TO USE ROCURONIUM
Rocuronium will be given to you by your anaesthetist. It is given to you intravenously either as a single injection or as a continuous infusion (over a longer period of time) into a vein.
The usual dose is 0.6 mg per kg body weight and its effect will last 30 to 40 minutes. During the surgery the effect of Rocuronium is controlled continuously.
If necessary, additional doses could be administered to you. The dose is adjusted to your needs by your anaesthetist. It depends on many factors, such as drug interactions (their cross activity), taking into consideration the estimated length of surgery as well as your age and clinical condition.
This medicinal product is for single use only. Paediatric use: For neonates (0 - 28 days), infants and toddlers (28 days-2 years), children (211 years) and adolescents (12 - 17 years) the recommended doses are similar to those in adults, with the exception of continuous infusion rates in children (2 - 11 years) which might be higher than in adults. The anaesthetist will adapt the infusion rate accordingly.
The experience with rocuronium bromide in a special type of anaesthetic technique called rapid sequence induction is limited in paediatric patients. Rocuronium bromide is therefore not recommended for this purpose in paediatric patients.
The following information is intended for healthcare professioi
For single use only.
Any unused solutions should be discarded.
The product should be used immediately after opening th
als only:
nium 10 mg/ml solution for injection / infusion has ; l (0.9%) and glucose 50 mg/ml (5%) solution.
ately flush
V004/GZ
If you receive more Rocuronlum than you should
Your anaesthetist will carefully monitor you when you are under medication of Rocuronium, therefore it is unlikely that you will be given too much Rocuronium. If it happens, your anaesthetist will make sure that anaesthesia and artificial ventilation will be continued until you breathe on your own.
Further questions
If you have any further questions on the use of this product, please ask your doctor or pharmacist.
For information intended for medicinal or heathcare professionals pease see accordant section below.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them. Hypersensitivity reactions (anaphylactic reaction/ shock) are very rare but may be life-threatening allergic reactions. A hypersenstivity reaction may include rash, itching, difficulty in breathing or swelling of the face, lips, throat or tongue.
Please inform your doctor or nurse immediately if one or more of these reactions occur. Uncommon/Rare (may affect up to 1 in 1,000 people):
• Pain at the injection site
• The drug is too effective, or not effective enough, or ineffective
• The drug works longer than expected (prolonged neuromuscular block)
• The drug prolongs the narcosis (delayed recovery from anesthesia)
• Lowering of blood pressure (hypotension)
• Increase in heart rate (tachycardia)
Very rare (may affect up to 1 in 10,000 peope):
• Increased level of histamine (mediator of allergic reactions) in the blood
• Wheezing (bronchospasm)
• Rash, itching
• Flushing
• Swelling of the face (facial oedema)
• Wide spread, severe rash (erythematous rash)
• Muscle weakness (myopathy)
• Welts (angioedema)
• Hives (urticaria)
• Loss of movement (flaccid paralysis)
• Failure of circulation (circulatory collaps and shock)
• Difficulty in breathing (respiratory failure, apnoea)
Not known (frequency cannot be estimated from the available data):
• Breathing (respiratory) failure
• Stop breathing (apnoea)
Paediatric patients
In clinical studies with rocuronium bromide in paediatric patients the side effect, increase in heart rate, was seen in up to 1 in 10 people.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly.
For UK - You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov uk/ yellowcard
For Ireland - Reports may be made by following the links to the online reporting option accessible from the IMB homepage, or by completing the downloadable report form also accessible from the IMB website, which may be completed manually and submitted to the IMB via freepost, to the following address:
FREEPOST
Pharmacovigilance Section Irish Medicines Board Kevin O'Malley House Earlsfort Centre Earlsfort Terrace Dublin 2
Tel: +353 1 6764971 Fax: +353 1 6762517
Website: www.imb.ie(http://www.imb.ie) e-mail: imbpharmacovigilance@imb.e
By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE ROCURONIUM
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after "EXP" The expiry date refers to the last day of that month. Store in a refrigerator (2-8°C).
Storage out of the refrigerator:
Rocuronium may also be stored outside of the refrigerator at a temperature of up to 30°C for a maximum of 12 weeks, after which it should be discarded. The product should not be placed back into the refrigerator, once it has been kept outside. The storage period must not exceed the shelf-life.
Manufacturer:
hameln pharmaceuticals gmbh
Langes Feld 13
31789 Hameln, Germany
This leaflet was last revised in Aug 2013.

The product should be used immediately after opening the vial.
After dilution: Chemical and physical in-use stability of a 5 mg/ml and 0.1 mg/ml solution (diluted with sodium chloride 9 mg/ml (0.9%) and glucose 50 mg/ml (5%) solution for infusion) has been demonstrated for 24 hours at room temperature. From the microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless dilution has taken place in controlled and validated aseptic conditions.
Do not use this medicine if you notice the solution is not clear or free from particles.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. FURTHER INFORMATION
What Rocuronium contains
The active substance is rocuronium bromide.
Each ml contains 10 mg of rocuronium bromide. Each 2.5 ml val contains 25 mg rocuronium bromide.
Each 5 ml vial contains 50 mg rocuronium bromide. Each 10 ml vial contain; 100 mg rocuronium bromide. The other ingredients are sodium acetate trihydrate, sodium chloride, glacial acetic acid 100% and water for injections.
What Rocuronium looks like and contents of the pack
Rocuronium is a dear, colouress to pale brownish-yellow solution for injection/infusion.
Pack size:
Rocuronium is available in packs of 5 or 10 vials containing 2.5 ml, 5 ml or 10 ml solution.
Not all pack sizes may be marketed.
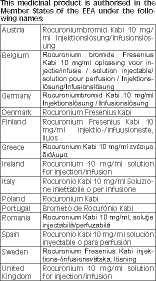
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Fresenius Kabi Limited
Cestrian Court
Eastgate Way
Manor Park
Runcorn
Cheshire
WA71NT
UK
Physic:
nlity
e, hydro
for Ro

V004/GZ