Ropivacaine 5 Mg/Ml Solution For Injection
PACKAGE LEAFLET: INFORMATION FOR THE USER
Ropivacaine 5 mg/ml solution for injection
Ropivacaine hydrochloride
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Ropivacaine is and what it is used for
2. Before you use Ropivacaine
3. How to use Ropivacaine
4. Possible side effects
5. How to store Ropivacaine
6. Further information
1. WHAT ROPIVACAINE IS AND WHAT IT IS USED FOR
Ropivacaine contains the active substance ropivacaine hydrochloride which is a type of medicine called local anaesthetic. These are chemical substances that are used to numb an area of the body.
Ropivacaine is used to numb (anaesthetise) specific parts of the body and to stop pain during surgery.
2. BEFORE YOU USE ROPIVACAINE
You must not be given Ropivacaine
- if you are allergic (hypersensitive) to ropivacaine hydrochloride,
other so called local anaesthetics of the amide type or any of the other ingredients of Ropivacaine,
- if you have a decrease in blood volume (hypovolaemia). This is measured by healthcare personnel.
- for injection into a blood vessel to numb a specific area of your body,
- for injection into the neck of the womb to relieve pain during childbirth.
Take special care with Ropivacaine
Special care should be taken to avoid any injection of Ropivacaine directly into a blood vessel to prevent any immediate toxic effects. Injection should not be performed in inflamed areas.
Please tell your doctor:
- if you are in a poor general conditon due to your age or other factors.
- if you have heart problems (partial or complete heart conduction block)
- if you have advanced liver problems
- if you have severe kidney problems.
Tell your doctor if you have any of these problems because your doctor may need to adjust the dose of Ropivacaine.
An injection into the lower part of your spine may induce low blood pressure or slow heart beat. If this occurs your doctor will initiate the appropriate measures.
Please tell your doctor:
- if you suffer from acute porphyria (problems with building up red blood pigment, sometimes resulting in neurological symptoms).
Tell your doctor if you or somebody in your family have porphyria because your doctor may need to use another anaesthetic.
Taking other medicines
Please tell your doctor or healthcare personnel if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Caution should be exercised if you are receiving:
- Other local anaesthetics (e.g. lidocaine) or agents structurally related to amide-type local anaesthetics, e.g. certain medicines used to treat an irregular heart beat (arrhythmia), such as mexiletine or amiodarone
- General anaesthetics or opioids, such as morphine or codeine
- Medicines used to treat depression (e.g. fluvoxamine)
- Certain antibiotics (e.g. enoxacin)
Pregnancy and breast-feeding
Before you are given Ropivacaine please tell your doctor if you are pregnant, planning to get pregnant, or if you are breastfeeding. It is not known if ropivacaine hydrochloride affects pregnancy or passes into breast milk.
Ask your doctor or pharmacist for advice before taking any medicine.
Paediatric patients
There is no experience with injection into the lower part of the spine (intrathecal administration), neither in infants nor in children of 12 years of age or younger.
Driving and using machines
Ropivacaine may make you feel sleepy and affect the speed of your reactions. After you have been given Ropivacaine, you should not drive or use any tools or machines until the next day.
Discuss with your doctor or pharmacist if you are unsure about anything.
Important information about some of the ingredients of Ropi-vacaine
This medicinal product contains 0.138 mmol (or 3.17 mg) sodium per ml. To be taken into consideration by patients on a controlled sodium diet.
3. HOW TO USE ROPIVACAINE
Method of administration
Your doctor will administer Ropivacaine to you. It is administered by injection.
Dosage
The dose used will depend on what it is being used for and also on your health, age and weight. The smallest dose that can produce effective numbing (anaesthesia) of the required area should be used. The usual dose
- for adults and adolescents older than 12 years of age is
between 15 mg and 25 mg of ropivacaine hydrochloride.
Duration of treatment
Administration of ropivacaine hydrochloride usually takes between 2 to 6 hours in case of anaesthesia prior to certain surgeries.
It is administered by injection into the lower part of your spine (intrathecal administration).
If you are given more Ropivacaine than you should be
The first symptoms of being given too much ropivacaine hydrochloride are usually problems with
- hearing and sight,
- numbness around the mouth,
- dizziness or light-headedness,
- tingling,
- speech disorder characterised by poor articulation (dysarthria),
- muscular stiffness, muscular twitching, fits (convulsions),
- low blood pressure,
- slow or irregular heart beat.
These symptoms may proceed to cardiac arrest, breathing arrest or severe fits.
The following information is intended for medical or healthcare professionals only:
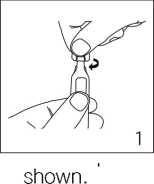
Handling
Ropivacaine should only be used by, or under the supervision of, clinicians experienced in regional anaesthesia (see section 3).
|
Concen tration mg/ml |
Volume ml |
Dose mg |
Onset minutes |
Duration hours | |
|
SURGICAL ANAESTHESIA Intrathecal Administration Surgery |
5.0 |
3-5 |
15-25 |
1-5 |
2-6 |
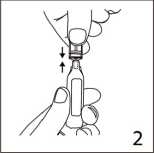
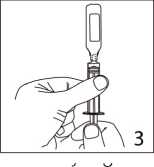
The ampoule fits both Luerfit and LuerLock syringes.
3. Hold the syringe with ampoule uppermost. Without squeezing the ampoule, withdraw the solution. Maintain downward pressure on the syringe piston after the solution has been withdrawn until the empty ampoule has been removed.
Shelf life
Shelf-life before opening 2 years
Shelf-life after opening
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8 °C.
Ropivacaine products are preservative free and are intended for single use only. Discard any unused solution.
The medicinal product should be visually inspected prior to use. The solution should only be used if it is clear, practically free from particles and if the container is undamaged.
The intact container must not be re-autoclaved.
Posology
Adults and adolescents (>12 years of age)
The following table is a guide to dosage for intrathecal block in adults. The smallest dose required to produce an effective block should be used. The clinician's experience and knowledge of the patient's physical status are of importance when deciding the dose.
The doses in the table are those considered to be necessary to produce a successful block and should be regarded as guidelines for use in adults. Individual variations in onset and duration occur. The figures in the column 'Dose' reflect the expected average dose range needed. Standard textbooks should be consulted for both factors affecting specific block techniques and individual patient requirements.
Method of administration
For intrathecal administration by injection only.
Careful aspiration before and during injection is recommended to prevent intravascular injection. An inadvertent intravascular injection may be recognised by a temporary increase in heart rate.
Aspiration should be performed prior to and during administration of the main dose, which should be injected slowly, at a rate of 25-50 mg/min, while closely observing the patient’s vital functions and maintaining verbal contact. If toxic symptoms occur, the injection should be stopped immediately.
The intrathecal injection should be made after the subarachnoid space has been identified and clear cerebrospinal fluid (CFS) is seen to escape from the spinal needle, or is detected by aspiration.
Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
In alkaline solutions precipitation may occur as ropivacaine hydrochloride shows poor solubility at pH > 6.0.
Disposal
Any unused product or waste material should be disposed of in accordance with local requirements.
If you experience any of these symptoms or think you may have received too much Ropivacaine, tell your doctor or healthcare personnel immediately.
In case of acute toxicity, appropriate corrective actions will be taken immediately by the healthcare personnel.
Due to the low dose administered during injection into the lower part of the spine (intrathecal administration), side effects affecting your whole body in general are not expected to occur.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Ropivacaine can cause side effects, although not everybody gets them.
The evaluation of side effects is based on the following frequencies:
|
very common: |
affects more than 1 user in 10 |
|
common: |
affects 1 to 10 users in 100 |
|
uncommon: |
affects 1 to 10 users in 1,000 |
|
rare: |
affects 1 to 10 users in 10,000 |
|
very rare: |
affects less than 1 user in 10,000 |
|
not known: |
frequency cannot be estimated from the available data |
Important side effects to look out for:
Sudden life-threatening allergic reactions (such as anaphylaxis, angioneurotic oedema and urticaria) are rare. Possible symptoms include
- sudden onset of rash,
- itching or lumpy rash (hives),
- swelling of the face, lips, tongue or other parts of the body,
- and shortness of breath, wheezing or difficulty breathing.
If you think that Ropivacaine is causing an allergic reaction, tell your doctor or healthcare personnel immediately.
Other possible side effects:
Very common
• Low blood pressure (hypotension). This might make you feel dizzy or light-headed.
• Feeling sick (nausea)
Common
• Headache, pins and needles (paraesthesia), feeling dizzy
• Slow or fast heart beat (bradycardia, tachycardia)
• High blood pressure (hypertension)
• Being sick (vomiting)
• Difficulty in passing urine (urinary retension)
• Back pain, increased temperature, muscular stiffness (rigor)
Uncommon
• Anxiety
• Some symptoms can happen if the injection was given into a blood vessel by mistake, or if you have been given too much Ropivacaine (see also “If you are given more Ropivacaine than you should be” above). These include fits (convulsions, seizures), feeling dizzy or light-headed, numbness of the lips and around the mouth, numbness of the tongue, hearing problems, problems with your sight (vision), problems with your speech (dysarthria), muscular twitching and trembling, reduced sense of touch (hypoaesthesia)
• Fainting (syncope)
• Difficulty breathing (dyspnoea)
• Low body temperature
Rare
• Cardiac arrest, irregular heart beat (cardiac arrhythmias)
Possible side effects seen with other local anaesthetics which might also be caused by Ropivacaine include:
• Numbness, due to nerve irritation caused by the needle or the injection. This does not usually last for long.
• Damaged nerves. Rarely, this may cause permanent problems.
• If too much Ropivacaine is given into the spinal fluid, the whole body may become numbed (anaesthetised).
Adolescents
In adolescents, the side effects are the same as in adults except for low blood pressure which happens less often in adolescents (affecting less than 1 in 10) and being sick which happens more often in adolescents (affecting more than 1 in 10). Ropivacaine should not to be used for injection into the lower part of the spine in infants and children younger than 12 years of age.
If any of the side effects get serious, or if you notice any side effects not listed in this leafl et, please tell your doctor or pharmacist.
5. HOW TO STORE ROPIVACAINE
Keep out of the reach and sight of children.
Do not use Ropivacaine after the expiry date which is stated on the ampoule or carton box. The expiry date refers to the last day of that month.
Do not freeze.
Do not use Ropivacaine if you notice any precipitation in the solution for injection.
Your doctor or the hospital will normally store Ropivacaine and they are responsible for the quality of the product when it has been opened if it is not used immediately. They are also responsible for disposing of any unused Ropivacaine correctly.
Medicines should not be disposed of via wastewater or household waste. Your doctor or pharmacist will dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What Ropivacaine contains
- The active substance is ropivacaine hydrochloride 5 mg/ml. Each 10 ml polypropylene ampoule contains 50 mg ropivacaine (as hydrochloride).
- The other ingredients are sodium chloride, sodium hydroxide (for pH adjustment) and water for injections.
What Ropivacaine looks like and contents of the pack
Ropivacaine solution for injection is a clear, colourless, sterile, isotonic, isobaric aqueous solution for injection.
Ropivacaine 5 mg/ml solution for injection is available in 10 ml transparent polypropylene ampoules.
Pack sizes:
10 ampoule(s) in plastic overwrap
Marketing Authorisation Holder and Manufacturer
Goldshield Pharmaceuticals Ltd NLA Tower
12-16 Addiscombe Rd
Croydon
CR0 0XT, UK