Salvacyl 11.25Mg Powder And Solvent For Suspension For Injection
Front Page


Package Leaflet: Information for the user
Salvacyl 11.25mg
powder and solvent for suspension for injection
Triptorelin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Salvacyl is and what it is used for
2. What you need to know before you use Salvacyl
3. How to use Salvacyl
4. Possible side effects
5. How to store Salvacyl
6. Contents of the pack and other information
Salvacyl contains triptorelin, which is similar to a hormone called gonadotropin releasing hormone (GnRH analogue). It is a long acting formulation designed to slowly deliver 11.25 mg of triptorelin over twelve weeks. It acts by lowering the levels of the male hormone, testosterone, in the body.
Salvacyl is used to decrease sexual drive in adult men with severe sexual deviations.
The treatment with Salvacyl is to be initiated and controlled by a psychiatrist. The treatment should be given in combination with psychotherapy, in order to decrease deviating sexual behaviour.
Do not use Salvacyl
• If you suffer from severe osteoporosis (a condition that weakens your bones).
• If you are allergic to triptorelin, other medicines that regulate the production and release of sex hormones, or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
At the beginning of treatment there will bean increased amount of testosterone in your body. This might result in an increase in sexual drive. Your doctor may give you some medicine (an anti-androgen) to control this effect.
Talk to your doctor:
• If you develop a depressed mood while taking Salvacyl. There have been reports of depression in patients taking Salvacyl which may be severe.
• If you are using anticoagulants (medicines that inhibit the blood clotting ability), since you may experience bruises at the site of injection.
• When Salvacyl is used for a long period of time, the risk of developing weak bones is increased, especially if any of the following applies: If you are a heavy drinker, a smoker, have osteoporosis (a condition that weakens your bones) or have a family history of osteoporosis, have a poor diet or take anticonvulsants (medicines for epilepsy or fits) or corticosteroids (steriods). In order to reduce the risk of brittle bones, a healthy lifestyle is recommended including no smoking, moderate alcohol intake and regular weight bearing exercises (e.g. walking, jogging or other sports that put a load on the skeleton and therefore strengthen it). You should make sure that your diet contains enough calcium and vitamin D.
• If you need any tests to check your hormone function during or after your Salvacyl treatment, the results may be misleading. Please tell your doctor you have been treated with Salvacyl.
• If you experience sudden headache, problems with eyesight and weakness of the eye muscles. These can be signs of a benign tumour of the pituitary gland which may be discovered following treatment with Salvacyl.
• If you have diabetes.
• If you have any heart or blood vessel conditions, including heart rhythm problems (arrhythmia), or are being treated with medicines for these conditions. The risk of heart rhythm problems may be increased when using Salvacyl.
When the treatment is stopped, testosterone will return to normal levels and your sexual drive may increase again. Your doctor may give you another medicine in order to control this effect.
Other medicines and Salvacyl
When Salvacyl is taken at the same time as medicines that affect the release of hormones from the pituitary (part of the brain), your doctor may need to check your hormone levels.
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Salvacyl might interfere with some medicines used to treat heart rhythm problems (e.g. quinidine, procainamide, amiodarone and sotalol) or might increase the risk of heart rhythm problems when used with some other drugs (e.g. methadone (used for pain relief and part of drug addiction detoxification), moxifloxacin (an antibiotic), antipsychotics used for serious mental illnesses).
Children
Salvacyl is not indicated for use in neonates, infants, children and adolescents.
#
Pregnancy and breast-feeding
Salvacyl is not to be used by women.
Driving and using machines
You may feel dizzy, tired or have problems with your sight such as blurred vision. These are possible side effects of treatment. If you experience any of these side effects you should not drive or use machines.
Salvacyl contains sodium
This medicinal product contains sodium but less than 1 mmol (23 mg) sodium per vial. This medicine is almost "sodium-free" and may betaken with a low sodium diet.
Salvacyl is prepared by and is always given to you by a doctor or nurse.
The usual dose is 11.25mg (one vial) of Salvacyl, given as a single injection into a muscle every twelve weeks. If you think the effect of Salvacyl is too strong or too weak, contact your doctor.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, Salvacyl can cause side effects, although not everybody gets them.
Many of the side effects are expected and are due to the change in the level of testosterone in your body. These effects include hot flushes and impotence.
In rare cases (may affect up to 1 in 1,000 people) you may experience a severe allergic reaction.Tell your doctor immediately if you develop symptoms such as swallowing or breathing problems, a rash, swelling of your lips, face, throat or tongue.
The following information is intended for healthcare professionals only (please see section 3):
Prepare the patient by disinfecting his gluteus at the injection site. This operation needs to be performed first because once reconstituted, the drug should be injected immediately.
The presence of bubbles on top of the lyophilisate is a normal appearance of the product.
• Take out the ampoule containing the solvent. Tap any solution within the tip of the ampoule back to the main body of the ampoule.
• Take out the vial containing the powder. Tap any powder which has accumulated at the top of the vial back to the bottom of the vial.
• Remove the plastic cap on top of the vial.
• Screw a needle onto the syringe (do not remove the needle cover yet).
• Break open the ampoule with dot face up.
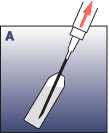
• Remove the needle protection from the needle. Insert the needle in the ampoule and draw up all of the solvent into the syringe.
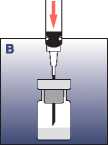
• Insert the needle through the rubber stopper vertically into the vial. Inject the solvent slowly, so that, if possible, it washes down the entire upper part of the vial.
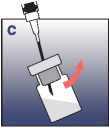
• Pull up the needle above the liquid level and reconstitute the suspension by swinging the vial gently, which means shaking horizontally.
Make sure that the agitation is long enough to obtain a homogeneous and milky suspension.
Salvacyl-Signes-11.25_mg-Leaflet-UK-1038862-(2.5).indd 1
5/13/15 3:12 PM


Very common: may affect more than 1 in 10 people:
• Hot flushes
• Excessive sweating
• Back pain
• Pins and needles sensation in the legs
• Loss of strength
Common: may affect up to 1 in 10 people:
• Nausea
• Tiredness; redness, inflammation, pain and/or other reactions at the injection site; muscle and bone pain; pain in the arms and legs; swelling caused by a build up of fluid in the body tissues
• Dizziness, headache
• Depression, mood changes
• Impotence
Uncommon: may affect up to 1 in 100 people:
Ringing in the ears
Pain in abdomen, constipation,
diarrhoea, vomiting
Drowsiness, shaking, sleepiness,
pain
• Some blood tests affected (including abnormal liver function tests)
• Increase in weight
• Loss of appetite, gout (severe pain and swelling in the joints,
usually in the big toe)
• Increase in appetite
• Joint pain, muscle cramps, muscle weakness, muscle pain
• Tingling or numbness
• Inability to sleep, irritability
• Development of enlarged breasts, breast pain, reduction in testicular size, pain in testicles
• Difficulty in breathing
• Acne, hair loss, itching, rash
• High blood pressure
Rare: may affect up to 1 in 1,000 people:
Feeling confused, decreased activity, having a feeling of elation or well-being Inability to ejaculate Shortness of breath when lying flat
Blisters
Nose bleeds
Low blood pressure
• Red or purple discolorations on the skin
• Vertigo (dizziness and a feeling •
• Abnormal sensation in the eye, blurring or disturbance in vision
• Sensation of fullness in the
stomach, flatulence, dry mouth, abnormal sense of taste Chest pain Difficulty in standing Flu-like illness, fever Allergic reaction, anaphylactic reaction (serious allergic reaction which can cause dizziness or difficulty in breathing)
Inflammation of the nose/throat Increase in an enzyme present in the bones and liver Increased body temperature Weight loss
Stiff joints, joint swelling, musculoskeletal stiffness, osteoarthritis Memory impairment
In studies and observations after marketing of Salvacyl, the following side effects have also been reported: Blurred vision, blood pressure increased, changes in ECG (QT prolongation), general discomfort, bone pain, anxiety, rapid formation of wheals due to swelling of the skin or mucous membranes.
As with other medicines of this class, an increase in white blood cell count may be found in patients being treated with Salvacyl.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.aov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Salvacyl
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after 'EXP'. The expiry date refers to the last day of that month.
Do not store above 25° C.
Chemical and physical in-use stability has been demonstrated for 24 hours at 25°C. From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Salvacyl contains
The active substance is triptorelin.
One vial of powder contains 11.25mg of triptorelin, as triptorelin embonate.
Reconstituted suspension (2mL) contains 11.25mg of triptorelin, as triptorelin embonate.
The other ingredients are:
Powder: poly (d, l-lactide-co-glycolide), mannitol, carmellose sodium and polysorbate 80. Solvent: water for injections.
#
What Salvacyl looks like and contents of the pack
Salvacyl is a white to off-white powder.
The solvent is a clear solution.
The package contains:
1 vial with powder 1 ampoule with 2mL of solvent
1 injection syringe
2 needles.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder Manufacturer
Ipsen Limited Ipsen Pharma Biotech
190 Bath Road Parc d'Activite du Plateau de signes
Slough, SL1 3XE Chemin Departemental n° 402
UK. 83870 Signes
France.
This leaflet was last revised in March 2015.
Is this leaflet hard to see or read? Please phone 01753 627777 and ask for help.
IP5EM
£ ^0459
vO
00
• Check there is no unsuspended powder in the vial (if any powder clumps are present, continue swirling until they disappear).
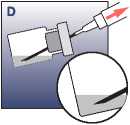
• When the suspension is homogeneous, pull down the needle and draw up all of the suspension (without inverting the vial). A small amount will remain in the vial and should be discarded. An overage is included to allow for this loss.
|
• Grasp the coloured hub to connect needle. Remove the needle used for the reconstitution from the syringe. Attach the other needle to the syringe tip (screw on tightly). • Remove the needle protection from the needle. |
u |
u |
|
3 - INJECT | ||
|
• Prime the needle to remove air from the syringe immediately before administrating the injection. To avoid precipitation, inject immediately into the gluteal muscle previously disinfected. |
p- | |
|
4-AFTER USE | ||
• Dispose of the needles in a designated sharps container.
Salvacyl-Signes-11.25_mg-Leaflet-UK-1038862-(2.5).indd 2
5/13/15 3:12 PM
1038862