Sandostatin Lar 30 Mg Powder And Solvent For Suspension For Injection
Package leaflet: Information for the patient
SANDOSTATIN LAR 10 mg powder and solvent for suspension for injection
SANDOSTATIN LAR 20 mg powder and solvent for suspension for injection
SANDOSTATIN LAR 30 mg powder and solvent for suspension for injection
octreotide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Sandostatin LAR is and what it is used for
2. What you need to know before you use Sandostatin LAR
3. How to use Sandostatin LAR
4. Possible side effects
5. How to store Sandostatin LAR
6. Contents of the pack and other information
1. What Sandostatin LAR is and what it is used for
Sandostatin LAR is a synthetic compound derived from somatostatin. Somatostatin is normally found in the human body, where it inhibits the release of certain hormones such as growth hormone. The advantages of Sandostatin LAR over somatostatin are that it is stronger and its effects last longer.
Sandostatin LAR is used
• to treat acromegaly,
Acromegaly is a condition where the body produces too much growth hormone. Normally, growth hormone controls growth of tissues, organs, and bones. Too much growth hormone leads to an increase in the size of bones and tissues, especially in the hands and feet. Sandostatin LAR markedly reduces the symptoms of acromegaly, which include headache, excessive perspiration, numbness of the hands and feet, tiredness, and joint pain. In most cases, the overproduction of growth hormone is caused by an enlargement in the pituitary gland (a pituitary adenoma); Sandostatin LAR treatment may reduce the size of the adenoma.
Sandostatin LAR is used to treat people with acromegaly:
- when other types of treatment for acromegaly (surgery or radiotherapy) are not suitable or haven't worked;
- after radiotherapy, to cover the interim period until the radiotherapy becomes fully effective.
• to relieve symptoms associated with overproduction of some specific hormones and other related substances by the stomach, bowels or pancreas,
Overproduction of specific hormones and other related natural substances can be caused by some rare conditions of the stomach, bowels or pancreas. This upsets the natural hormonal balance of the body and results in a variety of symptoms, such as flushing, diarrhoea, low blood pressure, rash, and weight loss. Treatment with Sandostatin LAR helps to control these symptoms.
• to treat neuroendocrine tumours located in the gut (e.g. appendix, small intestine or colon)
Neuroendocrine tumours are rare tumours which can be found in different parts of the body. Sandostatin LAR is also used to control the growth of these tumours, when they are located in the gut (e.g. appendix, small intestine or colon).
• to treat pituitary tumours that produce too much thyroid-stimulating hormone (TSH).
Too much thyroid-stimulating hormone (TSH) leads to hyperthyroidism. Sandostatin LAR is used to treat people with pituitary tumours that produce too much thyroid-stimulating hormone (TSH):
- when other types of treatment (surgery or radiotherapy) are not suitable or have not worked;
- after radiotherapy, to cover the interim period until the radiotherapy becomes fully effective.
2. What you need to know before you use Sandostatin LAR
Follow all instructions given to you by your doctor carefully. They may differ from the information contained in this leaflet.
Read the following explanations before you use Sandostatin LAR.
Do not use Sandostatin LAR:
- if you are allergic to octreotide or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor before using Sandostatin LAR:
- if you know that you have gallstones now, or have had them in the past; tell your doctor, as prolonged use of Sandostatin LAR may result in gallstone formation. Your doctor may wish to check your gallbladder periodically.
- if you know that you have diabetes, as Sandostatin LAR can affect blood sugar levels. If you are diabetic, your sugar levels should be checked regularly.
- if you have a history of vitamin B12 deprivation your doctor may wish to check your vitamin B12 level periodically.
Test and checks
If you receive treatment with Sandostatin LAR over a long period of time, your doctor may wish to check your thyroid function periodically.
Your doctor will check your liver function.
Children
There is little experience with the use of Sandostatin LAR in children. Other medicines and Sandostatin LAR
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
You can generally continue taking other medicines while on Sandostatin LAR. However, certain medicines, such as cimetidine, ciclosporin, bromocriptine, quinidine and terfenadine have been reported to be affected by Sandostatin LAR.
If you are taking a medicine to control your blood pressure (e.g. a beta blocker or a calcium channel blocker) or an agent to control fluid and electrolyte balance, your doctor may need to adjust the dosage.
If you are diabetic, your doctor may need to adjust your insulin dosage.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
Sandostatin LAR should only be used during pregnancy if clearly needed.
Women of child-bearing age should use an effective contraceptive method during treatment.
822^812
2184238_GB_p2_LFT.indd 1
|
(!) NOVARTIS |
Brewery House, The Maltings, y Silvester Street |y*^| Kingston-Upon-Hull >55^ The Maltings HU1 3HA Tel: +44 (0) 1482 973000 | |
|
Live Text: |
\x Yes / □ No / □ Both |
Production Site: |
|
WO: 1485863 |
CTM: | |
|
Comp. Description: Leaflet SANDOSTATIN LAR MPVI GB |
Printing Colours: PANTONE 314 C | |
|
Comp. Number New: 2184238 GB | ||
|
Comp. Number Old: 2161102 GB | ||
|
Format/Dimension: 594 x 210 mm | ||
|
Tech. Drawing No.: 799.4.9167/06 |
Technical Colours: Cutting Dimensions | |
|
Minimum Font Size: 9.0pt | ||
|
Font Type: News Gothic | ||
|
Proof Number: 2 03/03/2016 | ||
|
Braille: | ||
ERPRINTING ON !
2184238
Do not breast-feed while using Sandostatin LAR. It is not known whether Sandostatin LAR passes into breast milk.
Driving and using machines
Sandostatin LAR has no or negligible effects on the ability to drive and use machines. However, some of the side effects you may experience while using Sandostatin LAR, such as headache and tiredness, may reduce your ability to drive and use machines safely.
Sandostatin LAR contains sodium
Sandostatin LAR contains less than 1 mmol sodium (23 mg) per dose, which means it is essentially “sodium-free”.
3. How to use Sandostatin LAR
Sandostatin LAR must always be administered as an injection into the muscle of the buttocks. With repeated administration, the left and right buttock should be used alternately.
If you use more Sandostatin LAR than you should
No life-threatening reactions have been reported after overdose of Sandostatin LAR.
The symptoms of overdose are: hot flushes, frequent urination, tiredness, depression, anxiety and lack of concentration.
If you think that an overdose has happened and you experience such symptoms, tell your doctor straight away.
If you forget to use Sandostatin LAR
If your injection is forgotten, it is recommended that you are given it as soon as it is remembered, and then continue as usual. It will not do any harm if a dose is a few days late, but you could get some temporary re-appearance of symptoms until you get back on schedule.
If you stop using Sandostatin LAR
If you interrupt your treatment with Sandostatin LAR your symptoms may come back. Therefore, do not stop using Sandostatin LAR unless your doctor tells you to.
If you have any further questions on the use of this medicine, ask your doctor, nurse or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects could be serious. Tell your doctor straight away if you get any of the following:
Very common (may affect more than 1 in 10 people):
• Gallstones, causing sudden back pain.
• Too much sugar in the blood.
Instructions for preparation and intramuscular injection for Sandostatin LAR
FOR DEEP INTRAMUSCULAR INJECTION ONLY
Included in the injection kit:
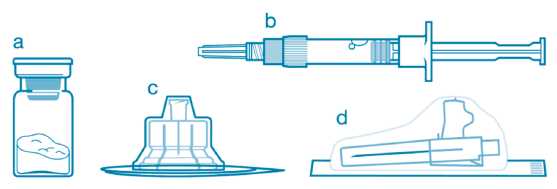
: a. One vial containing Sandostatin LAR powder,
b. One prefilled syringe containing the vehicle solution for reconstitution,
c. One vial adapter for drug product reconstitution, i d. One safety injection needle.
Follow the instructions below carefully to ensure proper reconstitution
■ of Sandostatin LAR before deep intramuscular injection.
: There are 3 critical actions in the reconstitution of Sandostatin LAR. Not ; following them could result in failure to deliver the drug appropriately. i • The injection kit must reach room temperature. Remove the ; injection kit from the fridge and let the kit stand at room : temperature for a minimum of 30 minutes before reconstitution,
but do not exceed 24 hours.
; • After adding the diluent solution, ensure that the powder is fully i saturated by letting the vial stand for 5 minutes. j • After saturation, shake the vial moderately in a horizontal direction ; for a minimum of 30 seconds until a uniform suspension is formed.
The Sandostatin LAR suspension must only be prepared : immediately before administration.
; Sandostatin LAR should only be administered by a trained healthcare i professional.
Step 1
■ • Remove the Sandostatin LAR injection ; kit from refrigerated storage.
: ATTENTION: It is essential to start the
reconstitution process only after the i injection kit reaches room temperature. Let j the kit stand at room temperature for a ; minimum of 30 minutes before ; reconstitution, but do not exceed 24 hours.
; Note: The injection kit can be re-refrigerated A if needed.
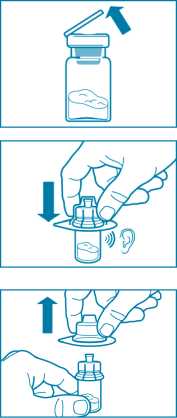
Step 2
• Remove the plastic cap from the vial and clean the rubber stopper of the vial with an alcohol wipe.
• Remove the lid film of the vial adapter packaging, but do NOT remove the vial adapter from its packaging.
• Holding the vial adapter packaging, position the vial adapter on top of the vial and push it fully down so that it snaps in place, confirmed by an audible “click.”
• Lift the packaging off the vial adapter with a vertical movement.
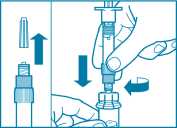
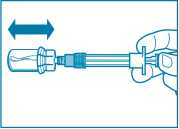
Step 3
• Remove the cap from the syringe prefilled with diluent solution and screw the syringe onto the vial adapter.
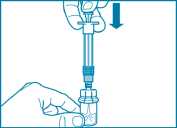
• Slowly push the plunger all the way down to transfer all the diluent solution in the vial.
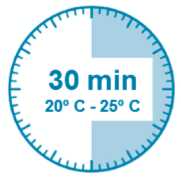
Step 4
ATTENTION: It is essential to let the vial stand for 5 minutes to ensure that the diluent has fully saturated the powder.
Note: It is normal if the plunger rod moves up as there might be a slight overpressure in the vial.
• At this stage prepare the patient for injection.
2184238 GB
03/03/2016 14:33
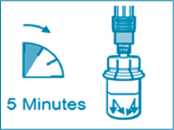
Step 5
• After the saturation period, make sure that the plunger is pushed all the way down in the syringe.
ATTENTION: Keep the plunger pressed and shake the vial moderately in a horizontal direction for a minimum of 30 seconds so that the powder is completely suspended (milky uniform suspension). Repeat moderate shaking for another 30 seconds if the powder is not completely suspended.
• Unscrew the syringe from the vial adapter.
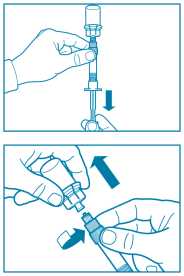
Step 6
• Turn syringe and vial upside down, slowly pull the plunger back and draw the entire contents from the vial into the syringe.
Step 8
• Sandostatin LAR must be given only by deep intramuscular injection, NEVER intravenously.
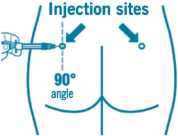
• Insert the needle fully into the left or right gluteus at a 90° angle to the skin.
• Slowly pull back the plunger to check that no blood vessel has been penetrated (reposition if a blood vessel has been penetrated).
• Depress the plunger with steady pressure until the syringe is empty. Withdraw the needle from the injection site
and activate the safety guard (as shown in Step 9).
Step 7
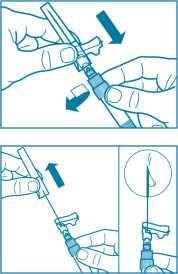
• Screw the safety injection needle onto the syringe.
• If immediate administration is delayed, gently re-shake the syringe to ensure a milky uniform suspension
• Prepare injection site with an alcohol wipe.
• Pull the protective cover straight off the needle.
• Gently tap the syringe to remove any visible bubbles and expel them from the syringe.
• Proceed immediately to Step 8 for administration to the patient. Any delay may result in sedimentation.
Step 9
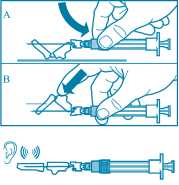
• Activate the safety guard over the needle in one of the two methods shown:
- either press the hinged section of the safety guard down onto a hard surface (figure A)
- or push the hinge forward with your finger (figure B).
• An audible “click” confirms the proper activation.
• Dispose of syringe immediately (in a sharps container).
Common (may affect up to 1 in 10 people):
• Underactive thyroid gland (hypothyroidism) causing changes in heart rate, appetite or weight; tiredness, feeling cold, or swelling at the front of the neck.
• Changes in thyroid function tests.
• Inflammation of the gallbladder (cholecystitis); symptoms may include pain in the upper right abdomen, fever, nausea, yellowing of the skin and eyes (jaundice).
• Too little sugar in the blood.
• Impaired glucose tolerance.
• Slow heart beat.
Uncommon (may affect up to 1 in 100 people):
• Thirst, low urine output, dark urine, dry flushed skin.
• Fast heart beat.
Other serious side effects
• Hypersensitivity (allergic) reactions including skin rash.
• A type of an allergic reaction (anaphylaxis) which causes difficulty in breathing or dizziness.
• An inflammation of the pancreas gland (pancreatitis); symptoms may include sudden pain in the upper abdomen, nausea, vomiting, diarrhoea.
• Liver inflammation (hepatitis); symptoms may include yellowing of the skin and eyes (jaundice), nausea, vomiting, loss of appetite, generally feeling unwell, itching, light-coloured urine.
• Irregular heart beat.
Tell your doctor straight away if you notice any of the side effects above. Other side effects:
Tell your doctor, pharmacist or nurse if you notice any of the side effects listed below. They are usually mild and tend to disappear as treatment progresses.
Very common (may affect more than 1 in 10 people):
• Diarrhoea.
• Abdominal pain.
• Nausea.
• Constipation.
• Flatulence (wind).
• Headache.
• Local pain at the injection site.
Common (may affect up to 1 in 10 people):
• Stomach discomfort after meal (dyspepsia).
• Vomiting.
• Feeling of fullness in the stomach.
• Fatty stools.
• Loose stools.
• Discolouration of faeces.
• Dizziness.
• Loss of appetite.
• Change in liver function tests.
• Hair loss.
• Shortness of breath.
• Weakness.
If you get any side effects, please tell your doctor, nurse or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly (see details below). By reporting side effects you can help provide more information on the safety of this medicine.
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
5. How to store Sandostatin LAR
Keep this medicine out of the sight and reach of children.
Store in the original package in order to protect from light.
Store in a refrigerator (2°C to 8°C). Do not freeze.
Sandostatin LAR may be stored below 25°C on the day of injection.
Do not store Sandostatin LAR after reconstitution (it must be used immediately).
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date refers to the last day of that month.
Do not use this medicine if you notice particles or a change of colour.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Sandostatin LAR contains
- The active substance is octreotide
One vial contains 10 mg, 20 mg or 30 mg octreotide (as octreotide acetate)
- The other ingredients are
in powder (vial): poly(DL-lactide-co-glycolide) and mannitol (E421). in solvent (prefilled syringe): carmellose sodium, mannitol (E421), poloxamer 188, water for injections
What Sandostatin LAR looks like and contents of the pack
Unit packs containing one 6 mL glass vial with rubber stopper (bromobutyl rubber), sealed with an aluminium flip-off seal,
containing powder for suspension for injection and one 3 mL colourless pre-filled glass syringe with front and plunger stopper (chlorobutyl rubber) with 2 mL solvent, co-packaged in a sealed blister tray with one vial adapter and one safety injection needle.
Multipacks of three unit packs, each unit pack containing: one 6 mL glass vial with rubber stopper (bromobutyl rubber), sealed with an aluminium flip-off seal, containing powder for suspension for injection and one 3 mL colourless pre-filled glass syringe with front and plunger stopper (chlorobutyl rubber) with 2 mL solvent, co-packaged in a sealed blister tray with one vial adapter and one safety injection needle.
Not all strengths and pack sizes may be marketed in your country.
This medicinal product is authorised in the Member States of the EEA under the following names:
Austria, Bulgaria, Croatia, Cyprus, Sandostatin LAR
Czech Republic, Denmark, Estonia, Finland,
Germany, Greece, Hungary, Ireland, Iceland,
Latvia, Lithuania, Malta, Norway, Poland,
Romania, Slovakia, Slovenia, Spain, Sweden,
United Kingdom
Belgium, Luxemburg, Netherlands Sandostatine LAR
Italy, Portugal Sandostatina LAR
France Sandostatine L.P.
Marketing Authorisation Holder and Manufacturer
Novartis Pharmaceuticals UK Limited, Frimley Business Park, Frimley, Camberley, Surrey, GU16 7SR, England.
This leaflet was last revised in 03/2016.
If you would like any more information, or would like the leaflet in a different format, please contact Medical Information at Novartis Pharmaceuticals UK Ltd, telephone number 01276 698370.
SANDOSTATIN is a registered trade mark Copyright Novartis Pharmaceuticals UK Limited
The following information is intended for healthcare professionals only:
How much Sandostatin LAR to use
Acromegaly
It is recommended to start treatment with the administration of 20 mg Sandostatin LAR at 4-week intervals for 3 months. Patients on treatment with s.c. Sandostatin can start treatment with Sandostatin LAR the day after the last dose of s.c. Sandostatin. Subsequent dosage adjustment should be based on serum growth hormone (GH) and insulin-like growth factor-1/somatomedin C (IGF-1) concentrations and clinical symptoms.
For patients in whom, within this 3-month period, clinical symptoms and biochemical parameters (GH; IGF-1) are not fully controlled
(GH concentrations still above 2.5 microgram/L), the dose may be increased to 30 mg every 4 weeks. If after 3 months, GH, IGF-1, and/or symptoms are not adequately controlled at a dose of 30 mg, the dose may be increased to 40 mg every 4 weeks.
For patients whose GH concentrations are consistently below 1 microgram/L, whose IGF-1 serum concentrations normalised, and in whom most reversible signs/symptoms of acromegaly have disappeared after 3 months of treatment with 20 mg, 10 mg Sandostatin LAR may be administered every 4 weeks. However, particularly in this group of patients, it is recommended to closely monitor adequate control of serum GH and IGF-1 concentrations, and clinical signs/symptoms at this low dose of Sandostatin LAR.
For patients on a stable dose of Sandostatin LAR, assessment of GH and IGF-1 should be made every 6 months.
Gastro-entero-pancreatic endocrine tumours
• Treatment of patients with symptoms associated with functional gastro-entero-pancreatic neuroendocrine tumours
It is recommended to start treatment with the administration of 20 mg Sandostatin LAR at 4-week intervals. Patients on treatment with s.c. Sandostatin should continue at the previously effective dosage for 2 weeks after the first injection of Sandostatin LAR.
For patients in whom symptoms and biological markers are well controlled after 3 months of treatment, the dose may be reduced to 10 mg Sandostatin LAR every 4 weeks.
For patients in whom symptoms are only partially controlled after 3 months of treatment, the dose may be increased to 30 mg Sandostatin LAR every 4 weeks.
For days when symptoms associated with gastro-entero-pancreatic tumours may increase during treatment with Sandostatin LAR, additional administration of s.c. Sandostatin is recommended at the dose used prior to the Sandostatin LAR treatment. This may occur mainly in the first 2 months of treatment until therapeutic concentrations of octreotide are reached.
• Treatment of patients with advanced Neuroendocrine Tumours of the midgut or of unknown origin where non-midgut sites of origin have been excluded
The recommended dose of Sandostatin LAR is 30 mg administered every 4 weeks. Treatment with Sandostatin LAR for tumour control should be continued in the absence of tumour progression.
Treatment of TSH-secreting adenomas Treatment with Sandostatin LAR should be started at a dose of 20 mg at 4-weekly intervals for 3 months before considering dose adjustment. The dose is then adjusted on the basis of the TSH and thyroid hormone response.
2184238 GB
2184238_GB_p2_LFT.indd 2
03/03/2016 14:33
|
lb NOVARTIS |
Brewery House, The Maltings, Cf Silvester Street lOj Kingston-Upon-Hull The Maltings HU1 3HA Tel: +44 (0) 1482 973000 | |
|
Live Text: [X Yes / □ No / □ Both |
Production Site: | |
|
WO: 1485863 |
CTM: | |
|
Comp. Description: Leaflet SANDOSTATIN LAR MPVI GB |
Printing Colours: PANTONE 314 C | |
|
Comp. Number New: 2184238 GB | ||
|
Comp. Number Old: 2161102 GB | ||
|
Format/Dimension: 594 x 210 mm | ||
|
Tech. Drawing No.: 799.4.9167/06 |
Technical Colours: Cutting Dimensions | |
|
Minimum Font Size: 9.0pt | ||
|
Font Type: News Gothic | ||
|
Proof Number: 2 03/03/2016 | ||
|
Braille: | ||
|
! PLEASE TURN OVERPRINTING ON ! | ||
730166_0