Sativex Oromucosal Spray
o use Sativex
BeeharryN a 110:14 am, Juro 27, 2016
26.05.16[2]
Sativex® Oromucosal Spray
(delta-9-tetrahydrocannabinol and cannabidiol)
Your medicine will be referred to as Sativex throughout this leaflet.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Sativex is and what it is used for
2. What you need to know before you use Sativex
3. How to use Sativex
4. Possible side effects
5. How to store Sativex
6. Contents of pack and other information
1. What Sativex is and what it is used for What Sativex is
Sativex is a mouth spray which contains cannabis extracts called cannabinoids.
What Sativex is used for
Sativex is used in multiple sclerosis (MS) to improve symptoms related to muscle stiffness. This is also called “spasticity”. Spasticity means there is an increase in 'muscle tone' which makes the muscles feel more stiff or rigid. This means it is more difficult than normal to move the muscle. Sativex is used when other medicines have not helped your muscle stiffness.
Your 4 week trial of Sativex
Only a specialist doctor can start you on treatment with Sativex.
• Before you start using Sativex your specialist doctor will do an assessment. This is to see how bad your muscle stiffness is. They will look at how well other treatments have worked.
• You will then have a 4 week trial of Sativex. After this, your specialist doctor will do another assessment to see whether Sativex is helping you.
• Only if you have shown a significant improvement in your spasticity related symptoms after these 4 weeks should you continue to be treated with Sativex.
2. What you need to know before you use Sativex
Do not use Sativex:
• If you are allergic to cannabis extracts or any of the other ingredients of this medicine (listed in section 6).
• If you or anyone directly related to you has any mental health problems such as schizophrenia, psychosis or other significant psychiatric disorder. This does not include depression due to your multiple sclerosis.
• If you are breast-feeding.
Do not use this medicine if any of the above apply to you. If you are not
sure, talk to your doctor or pharmacist before using Sativex.
Warnings and precautions
Talk to your doctor or pharmacist before using Sativex:
• If you are pregnant or plan to become pregnant.
• Whether male or female you must use a reliable contraceptive method while using this medicine (see also “Pregnancy, breast-feeding and contraception (men and women)”', below).
• If you are under 18 years of age.
• If you have epilepsy or regular fits (seizures).
• If you have liver or kidney problems.
• If you have a serious heart problem such as angina, a previous heart attack, poorly controlled high blood pressure or a problem with your heart rate or heart beat.
• If you are elderly, especially if you have problems doing everyday activities such as making hot food and drinks.
• If you have previously abused any drug or substance.
If any of the above apply to you (or you are not sure), talk to your doctor
or pharmacist before using Sativex.
■way some other medicines work. Also, some other medicines can affect the way Sativex works.
In particular, tell your doctor or pharmacist if you are using any of the following medicines:
• Medicines that reduce anxiety (sedatives) or make you sleep better (hypnotics). These medicines may increase the side effects of Sativex and may increase the risks of falls and other accidents.
• Medicines to relax your muscles such as baclofen or diazepam. This is because taking Sativex with these medicines may increase the risk of you falling over.
If any of the above apply to you (or you are not sure), talk to your doctor or pharmacist before using Sativex.
If you see a different doctor or go into hospital, let them know all the medicines you are using.
Sativex with food, drink and alcohol
• In general, alcoholic beverages should be avoided whilst using Sativex especially at the beginning of treatment or when changing dose. If you do drink alcohol while using Sativex, be aware that using Sativex and alcohol together may increase their effects (such as loss of balance or ability to respond quickly) which could increase the risk of falls and other accidents.
• You can use Sativex with or without food (but see section 3 below “How to use Sativex”).
Pregnancy, breast-feeding and contraception (men and women)
• If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
• Do not use Sativex during pregnancy, unless advised to by your doctor.
• Whether male or female you must use a reliable contraceptive method while using this medicine. Keep using it for at least 3 months after your treatment has stopped.
• Do not use Sativex while breast-feeding.
If you are pregnant or breast-feeding always ask your doctor for advice
before taking any medicine.
Driving and using machines
• You must not drive or use machinery when you first start to take Sativex and until you are established on a stable daily dose.
• Sativex may cause you to feel sleepy or dizzy, which may impair your judgment and performance of skilled tasks. It has also rarely been reported to cause a brief loss of consciousness.
• Once you are more used to taking Sativex and your dose is stable, you should still not drive or use machinery if Sativex causes effects such as sleepiness or dizziness that could impair your ability to perform these tasks. If you are not sure, do not drive or operate machines.
The medicine can affect your ability to drive as it may make you sleepy or dizzy.
• Do not drive while taking this medicine until you know how it affects you.
• It is an offence to drive if this medicine affects your ability to drive.
• However, you would not be committing an offence if:
o The medicine has been prescribed to treat a medical or dental problem and
o You have taken it according to the instructions given by the prescriber or in the information provided with the medicine and o It was not affecting your ability to drive safely Talk to your doctor or pharmacist if you are not sure whether it is safe for you to drive while taking this medicine.
Foreign travel with Sativex
Before going abroad, check that it is legal for you to take this medicine. This includes any countries you are travelling through.
• Sativex is a controlled drug and its legal status will vary between countries.
• Driving while taking Sativex might be illegal in some countries.
• Please refer to the end of the leaflet for additional information on foreign travel.
Sativex contains ethanol and propylene glycol
• Sativex contains about 50% v/v of ethanol (alcohol) i.e. up to 40 mg per dose. The amount of alcohol in the maximum daily dose for most people (12 sprays) is about the same as found in two teaspoons (10 ml) of beer and about one teaspoon (5 ml) of wine. This product can be harmful for those suffering from alcoholism.
• Sativex contains propylene glycol which may cause irritation.
Symptoms of irritation are pain, itchiness, dry cough, sore throat.
3. How
Always
doctor
sure.
Only use Sativex in your mouth - on the inside of your cheek or under your tongue.
• You can take Sativex with or without food. However, taking Sativex with food can affect the amount your body takes in. You should try, as far as possible, to take Sativex the same way in relation to food each time, so you get the same effect each time.
Opening your spray and getting it ready to use
1. Take your spray out of the refrigerator (see section 5 for important information on Storing Sativex).
2. Write the date that you open your spray on the spray container label. Do not use the spray after it has been open for more than 6 weeks (42 days).
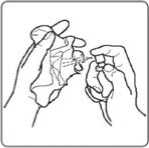
3. Shake the spray container gently before use.
4. Remove the protective cap.
5. Hold the spray between your thumb and second finger. Put your first finger on the nozzle.
6. Hold the spray upright, then practice spraying into a tissue 2 or 3 times until a fine spray appears. These sprays “prime” the pump and make sure it is working properly.
7. The spray is now ready to use. You will not need to do any more priming sprays until you open a new spray container.
Using your spray
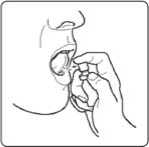
1. Hold the spray between your thumb and second finger. Put your first finger on the nozzle.
2. Hold it upright and point into your mouth.
Point the nozzle under your tongue or onto the inside of your cheek. Change the area in your mouth where you spray each time. This helps to stop any discomfort in one place.
3. Press the nozzle down firmly. Do not take more than one spray at a time, even if you feel that you only got a small amount of spray.
4. Replace the protective cap.
If you get spray in your eyes by accident, wash them as soon as possible
with water.
• Do not breathe in the spray.
• Do not spray near children or pets.
• Do not use the spray near naked flames or heat sources.
Working out how much to use
The number of sprays you need each day depends on you as an
individual. Each person needs a different number of sprays to give them
the best relief from their muscle stiffness, with the fewest unwanted
effects.
• When you first start using Sativex, you need to follow the days and times in the following table until you find the best number of sprays for you.
• Stop increasing your sprays when you find the best number of sprays for you. This may only take a few days or it may take up to 2 weeks. Aim to use this number of sprays each day. You can then spread your sprays evenly over the whole day.
• Do not use more than one spray at a time. Always leave at least 15 minutes between sprays.
• Do not over-exert yourself during the first couple of days of using Sativex until you know how it affects you.
• If you start to feel unwanted effects (usually dizziness) use one less spray each day until you find the best symptom relief with the fewest unwanted effects.
• When you find the best number of sprays for you, aim to use this number each day. You can then spread your sprays out evenly over the whole day, in a way that suits you. Still leave at least 15 minutes between sprays.
Other medicines and Sativex
Tell your doctor or pharmacist if you are using or have recently used or might use any other medicines. This is because Sativex may affect the
|
Number of sprays | |||
|
Morning (between waking-up and 12 noon) |
Evening (between 4 pm and bedtime) |
Total sprays each day | |
|
Day 1 |
0 |
1 |
1 |
|
Day 2 |
0 |
1 |
1 |
|
Day 3 |
0 |
2 |
2 |
|
Day 4 |
0 |
2 |
2 |
|
Day 5 |
1 |
2 |
3 |
|
Day 6 |
1 |
3 |
4 |
|
Day 7 |
1 |
4 |
5 |
|
Day 8 |
2 |
4 |
6 |
|
Day 9 |
2 |
5 |
7 |
|
Day 10 |
3 |
5 |
8 |
|
Day 11 |
3 |
6 |
9 |
|
Day 12 |
4 |
6 |
10 |
|
Day 13 |
4 |
7 |
11 |
|
Day 14 |
5 |
7 |
12 |
PL 20636/2870
26.05.16[2]
Do not use more than 12 sprays in one day, unless your doctor tells you to.
If you use more Sativex than you should
If you accidentally use more of this medicine than you normally do you may:
• See or hear things that are not there (hallucinations).
• Feel dizzy, sleepy or confused.
• Feel your heart rate change.
• Please tell your doctor or pharmacist if you used more Sativex than you should.
If you forget to use Sativex
• If you forget a dose, use a spray as soon as you remember or when you feel you need a spray.
• Do not use 2 sprays at the same time to make up for a missed spray. Knowing if your spray is nearly empty
After the 3 priming sprays, your spray contains up to 90 measured sprays. When the spray is becoming empty, the noise of the spray action may change. You may also find the spray feels different in your mouth. This is because your spray is nearly empty. When this happens you should open a new spray container.
If you stop using Sativex
If for any reason you decide to stop using Sativex, tell your doctor or pharmacist. If you stop using your medicine suddenly your sleep, appetite or feelings might be affected for a short time. Your muscle stiffness usually comes back gradually if you stop using Sativex.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. These are more likely when you start your treatment. In most cases side effects are quite mild and they generally wear off within a few days, please use less sprays or stop using Sativex until you feel normal again. When you start using the medicine again, go back to the number of sprays where you did not feel these unwanted effects.
If you get any side effects, talk to your doctor or pharmacist. This includes any side effects not listed in this leaflet.
If you get any of the following side effects please stop taking the medicine and contact your doctor:
• Difficulty speaking.
• Blurred vision.
• Problems with your memory or having trouble concentrating.
• Feeling over excited or losing touch with reality.
• Feeling depressed or confused.
• Seeing or hearing things that are not there (hallucinations).
• Believing ideas that are not true.
• Feeling that other people are against you.
• Thoughts of suicide.
• Changes in pulse rate, heart rate or blood pressure.
Very common (affecting more than 1 in 10 people)
• Feeling dizzy or tired.
Common (affecting less than 1 in 10 people)
• Feeling sleepy or giddy.
• Eating more or less than usual.
• Changed sense of taste or a dry mouth.
• Constipation or diarrhoea.
• Feeling or being sick.
• Mouth problems, including burning, pain or mouth ulcers.
• Lack of energy or feeling weak or generally unwell.
• Feeling abnormal or drunk.
• Loss of balance or falling over.
Uncommon (affecting less than 1 in 100 people)
• Fainting.
• Sore throat or throat irritation.
• Tummy pain.
• Mouth or teeth changing colour.
• Irritation where Sativex is sprayed.
• Red and swollen mouth or peeling inside it. Do not keep spraying onto these areas.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Sativex
• Keep this medicine out of the sight and reach of children.
• Do not use this medicine after the expiry date shown on the product packaging. The expiry date refers to the last day of that month.
• Store unopened Sativex upright in its carton in a refrigerator (2oC to 8o C). If it is not stored in a refrigerator it will become unstable and is unlikely to work.
• Store opened Sativex in an upright position and do not store above 25oC.
• Do not use Sativex after it has been open for 42 days.
• If the spray becomes discoloured or shows any other signs of deterioration, consult your pharmacist who will tell you what to do.
• Medicines should not be disposed via wastewater or household waste.
If your doctor decides to stop treatment, return the inhaler to your doctor or pharmacist for safe disposal. These measures will help to protect the environment.
6. Contents of pack and other information What Sativex contains
• The active substances are cannabis extracts.
Each millilitre (ml) contains 38- 44 mg and 35-42 mg of two extracts (as soft extracts) from Cannabis sativa L., leaf and flower, corresponding to 27 mg/ml delta-9-tetrahydrocannabinol (THC) and 25 mg/ml cannabidiol (CBD).
Each 100 microlitre spray contains 2.7 mg THC and 2.5 mg CBD.
• Also contains 50% v/v ethanol, propylene glycol and peppermint oil.
What Sativex looks like and contents of the pack
Sativex is a yellow/brown liquid in a 10 ml glass spray container with a pump. The pump is protected with a plastic cap. The number of measured sprays in the container is up to 90 sprays (after 3 priming sprays).
Sativex is packed as 3 spray containers in each carton.
Manufacturer and Product Licence holder
Manufactured by GW Pharma Limited, Salisbury, Wiltshire, SP4 0JQ, UK. Procured from within the EU by Product Licence holder Star Pharmaceuticals Ltd., 5 Sandridge Close, Harrow, Middlesex, HA1 1XD. Repackaged by Servipharm Ltd.
POM
Leaflet Revision date Ref:
Sativex is a trademark of GW Pharma Ltd.
Foreign travel with Sativex
Check that it is legal for you to take this medicine into any countries you are travelling to and countries you are travelling through. Sativex is a Controlled Drug and therefore its legal status will vary between countries. Driving while taking Sativex might be illegal in some countries.
When you leave the United Kingdom, you will not need a Home Office licence if you are travelling for less than three months or carrying no more than three months supply of medicine. However, you will need to carry a letter from your doctor that has your name, address and date of birth on it. It will need to show outward and return dates of travel, the countries you are visiting and the medicine you are carrying. It will need to detail the strength of the medicine and the total amount you have with you. When you travel abroad, contact the Embassy/Consulate/High Commission of the country you are travelling to for the rules for that country concerning Controlled Drugs. Information on these is given at: http://homeoffice.gov.uk/drugs/licensing/personal/