Sayanaject 104 Mg Suspension For Injection
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
SAYANAJECT 104 mg suspension for injection
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
SAYANAJECT single-dose container with 104 mg medroxyprogesterone acetate (MPA) in 0.65 ml suspension for injection.
Each pre-filled injector contains 104 mg medroxyprogesterone acetate (MPA). Excipients with known effect:
Methyl parahydroxybenzoate (E218) - 1.04 mg per 0.65 ml Propyl parahydroxybenzoate (E216) - 0.0975 mg per 0.65 ml Sodium - 2.47 mg per 0.65 ml
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Suspension for injection
White to off-white homogeneous suspension
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
SAYANAJECT is indicated for long-term female contraception. Each subcutaneous injection prevents ovulation and provides contraception for at least 13 weeks (+/- 1 week). However, it should be taken into consideration that the return to fertility (ovulation) may be delayed for up to one year (see section 4.4).
Since loss of bone mineral density (BMD) may occur in females of all ages who use SAYANAJECT long-term (see section 4.4), a risk/benefit assessment, which also takes into consideration the decrease in BMD that occurs during pregnancy and/or lactation, should be performed before administration of SAYANAJECT.
It is also important that the patient is informed about the long-term nature of this product’s effects, including a delayed return to fertility (see section 4.4).
Use in Adolescents (12-18 years)
In adolescents, use of SAYANAJECT is only indicated when other contraceptive methods are considered unsuitable or unacceptable, due to unknown long-term effects of bone loss associated with SAYANAJECT during the critical period of bone accretion (see section 4.4).
SAYANAJECT has not been studied in women under the age of 18 years but data is available for intramuscular MPA in this population.
4.2 Posology and method of administration
Sayanaject may be administered by a healthcare professional (HCP) or when considered appropriate by the HCP, self-injected by the patient, with medical follow up as necessary in accordance with local clinical guidance.
Administration of Sayanaject should be initiated under the supervision of a healthcare professional (HCP). After proper training in injection technique and schedule of administration, patients may self-inject with Sayanaject if their HCP determines that it is appropriate and with medical follow-up as necessary.
The Sayanaject single-dose container should be at room temperature. It must be vigorously shaken just before use to ensure that the dose being given represents a uniform suspension.
The contents are completely sealed inside the reservoir of the injector. The injector must be activated before use. The activation process pierces an internal seal so that the medicine can come out through the needle when the reservoir is squeezed. The liquid does not completely fill the reservoir. There is a small bubble of air above the liquid. The dose is administered as a subcutaneous injection (SC) into the anterior thigh or abdomen. When the injection is being given, the injector must be used with the needle downwards. This ensures that the full dose of liquid is delivered out through the needle. The medication should be injected slowly for 5-7 seconds.
Mixing the medicine
• Ensure that the Sayanaject single-dose container is at room temperature.
• Hold the injector firmly by the port.
• Shake the injector vigorously for at least 30 seconds to mix the medicine.
The medicine should appear white and uniform. If it is not, discard the injector and use a new one.
If you see liquid leaking out or any other problem, discard the injector and use a new one.
If there is a delay before injecting, you must repeat the mixing step.
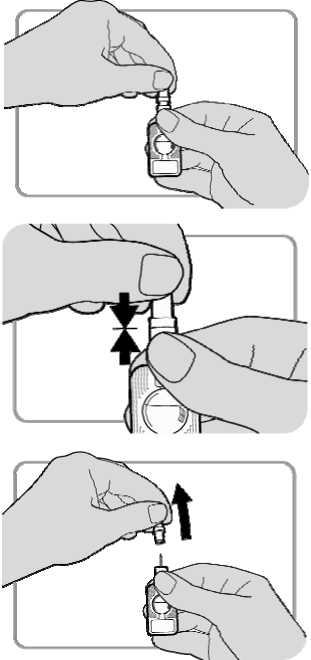
Activating the injector
• Hold the injector firmly by the port, making sure the needle shield is pointing upwards. Take care not to squeeze the reservoir.
• Hold the needle shield with the other hand.
• Push the needle shield firmly towards the port until it will go no further. The injector is now activated.
• Pull the needle shield off, and discard it.
Please refer to the Instructions for Use included with the Patient Leaflet for full details on preparing and giving an injection.
Adults
First Injection: To provide contraceptive cover in the first cycle of use, an injection of 104 mg SC should be given during the first five days of a normal menstrual cycle. If the injection is carried out according to these instructions, no additional contraceptive measure is required.
Further doses: The second and subsequent injections should be given at 13 week intervals, as long as the injection is given no later than seven days after this time, no additional contraceptive measures (e.g. barrier) are required. If the interval from the preceding injection is greater than 14 weeks (13 weeks plus 7 days) for any reason, then pregnancy should be excluded before the next injection is given. The efficacy of Sayanaject depends on adherence to the recommended dosage schedule of administration.
Women should be re-evaluated periodically as clinically appropriate at least every year to determine if Sayanaject is still the best option for them.
PostPartum: If the patient is not breast-feeding, the injection should be given within 5 days post partum (to increase assurance that the patient is not pregnant). If the injection is to be given at another time then the pregnancy should be excluded.
If the patient is breast-feeding, the injection should be given no sooner than six weeks post partum, when the infant’s enzyme system is more developed (see section 4.6).
There is evidence that women prescribed Sayanaject in the immediate puerperium can experience prolonged and heavy bleeding. Because of this, the drug should be used with caution in the puerperium. Women who are considering use of the product immediately following delivery or termination should be advised that the risk of heavy or prolonged bleeding may be increased. Doctors are reminded that in the non breast-feeding, post partum patient, ovulation may occur as early as week 4.
Switching from other Methods of Contraception: When switching from other contraception methods, Sayanaject should be given in a manner that ensures continuous contraceptive coverage based upon the mechanism of action of both methods, (e.g. patients switching from oral contraceptives should have their first injection of Sayanaject within 7 days after their last active pill).
Hepatic impairment: The effect of hepatic disease on the pharmacokinetics of Sayanaject is unknown. As Sayanaject largely undergoes hepatic elimination it may be poorly metabolised in patients with severe hepatic insufficiency (see section 4.3).
Renal impairment: The effect of renal disease on the pharmacokinetics of Sayanaject is unknown. No dosage adjustment should be necessary in women with renal insufficiency, since Sayanaject is almost exclusively eliminated by hepatic metabolism.
Paediatric population
Sayanaject is not indicated before menarche (see section 4.1). Data in adolescent females (12-18 years) is available for IM administration of MPA (see section 4.4 and section 5.1). Other than concerns about loss of BMD, the safety and effectiveness of Sayanaject is expected to be the same for adolescents after menarche and adult females.
4.3 Contraindications
Medroxyprogesterone acetate is contra-indicated
• in patients with a known hypersensitivity to MPA or any of its excipients listed in section 6.1.
• if pregnancy is known or suspected.
• in women with known or suspected malignancy of the breast or genital organs.
• in patients with undiagnosed vaginal bleeding.
• in patients with severe hepatic impairment.
• in patients with metabolic bone disease.
• in patients with active thromboembolic disease and in patients with current or past history of cerebrovascular disease.
4.4 Special warnings and precautions for use
Warnings:
Loss of Bone Mineral Density:
Use of depot MPA subcutaneous injection reduces serum estrogen levels and is associated with significant loss of BMD due to the known effect of estrogen deficiency on the bone remodelling system. Bone loss is greater with increasing duration of use, however BMD appears to increase after DMPA subcutaneous injection is discontinued and ovarian estrogen production increases.
This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. It is unknown if use of DMPA subcutaneous injection by younger women will reduce peak bone mass and increase the risk for fracture in later life.
A study to assess the BMD effects of medroxyprogesterone acetate IM (Depo-Provera, DMPA) in adolescent females showed that its use was associated with a significant decline in BMD from baseline. In the small number of women who were followed-up, mean BMD recovered to around baseline values by 1- 3 years after discontinuing treatment. In adolescents, SAYANAJECT may be used, but only after other methods of contraception have been discussed with the patients and considered to be unsuitable or unacceptable.
In women of all ages, careful re-evaluation of the risks and benefits of treatment should be carried out in those who wish to continue use for more than 2 years. In particular, in women with significant lifestyle and/or medical risk factors for osteoporosis, other methods of contraception should be considered prior to use of SAYANAJECT.
Significant risk factors for osteoporosis include:
Alcohol abuse and/or tobacco use
Chronic use of drugs that can reduce bone mass, e.g., anticonvulsants or corticosteroids
Low body mass index or eating disorder, e.g., anorexia nervosa or bulimia Previous low trauma fracture Family history of osteoporosis
A retrospective cohort study using data from the General Practice Research Database (GPRD) reported that women using MPA injections (DMPA), have a higher risk of fracture compared with contraceptive users with no recorded use of DMPA (incident rate ratio 1.41, 95% CI 1.35-1.47 for the five year follow-up period); it is not known if this is due to DMPA, or to other related lifestyle factors which have a bearing on fracture rate. By contrast, in women using DMPA, the fracture risk before and after starting DMPA was not increased (relative risk 1.08, 95% CI 0.92-1.26). Importantly, this study could not determine whether use of DMPA has an effect on fracture rate later in life.
For further information on BMD changes in both adult and adolescent females, as reported in recent clinical studies, refer to section 5.1 (Pharmacodynamic Properties). Adequate intake of calcium and Vitamin D, whether from the diet or from supplements, is important for bone health in women of all ages.
Menstrual Irregularities:
Most women using DMPA subcutaneous injection experienced alteration of menstrual bleeding patterns. Patients should be appropriately counseled concerning the likelihood of menstrual disturbance and the potential delay in return to ovulation. As women continued using DMPA subcutaneous injection, fewer experienced irregular bleeding and more experienced amenorrhea. After receiving the fourth dose, 39% of women experienced amenorrhea during month 6. During month twelve, 56.5% of women experienced amenorrhea. The changes in menstrual patterns from the three contraception trials are presented in Figures 1 and 2. Figure 1 shows the increase in the percentage of women experiencing amenorrhea over the 12 month study. Figure 2 presents the percentage of women experiencing spotting only, bleeding only, and bleeding and spotting over the same time period. In addition to amenorrhea, altered bleeding patterns included intermenstrual bleeding, menorrhagia and metrorrhagia. If abnormal bleeding associated with DMPA subcutaneous injection persists or is severe, appropriate investigation and treatment should be instituted.
Figure 1. Percent of DMPA subcutaneous injection -Treated Women with
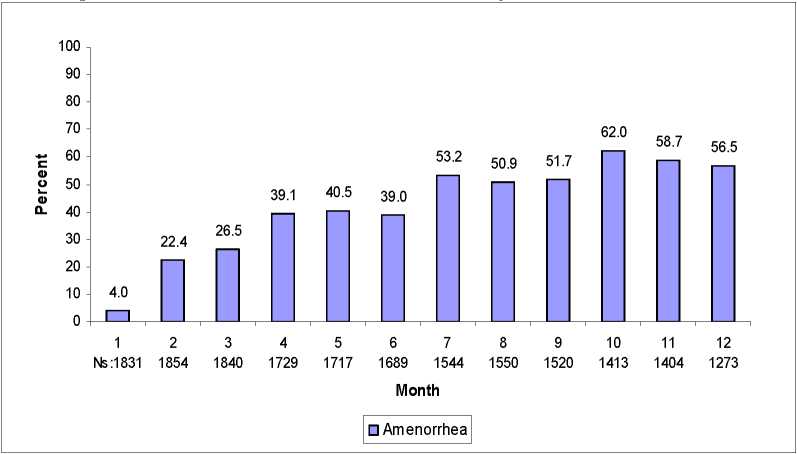
Figure 2. Percent of DMPA subcutaneous injection -Treated Women with Bleeding and/or Spotting per 30-Day Month Contraception Studies (ITT Population, N=2053) 100
90
80
70
v
o
V
0.
60
50
40
30
20
Cancer Risks:
Long-term case-controlled surveillance of DMPA-IM 150 mg users found no overall increased risk of ovarian, liver, or cervical cancer and a prolonged, protective effect of reducing the risk of endometrial cancer in the population of users.
Breast cancer is rare among women under 40 years of age whether or not they use hormonal contraceptives.
Results from some epidemiological studies suggest a small difference in the risk of having the disease_in current and recent users compared with never-users. Any excess risk in current and recent DMPA users is small in relation to the overall risk of breast cancer, particularly in young women (see below), and is not apparent after 10 years since last use. Duration of use does not seem to be important.
Possible number of additional cases of breast cancer diagnosed up to 10 years after stopping injectable progestogens*
|
Age at last use of DMPA |
No of cases per 10,000 women who are never-users |
Possible additional cases per 10,000 DMPA users |
|
20 |
Less than 1 |
Much less than 1 |
|
30 |
44 |
2-3 |
40
160
10
*based on use for 5 years”
Thromboembolic Disorders
Although MPA has not been causally associated with the induction of thrombotic or thromboembolic disorders, any patient who develops such an event, e.g. pulmonary embolism, cerebrovascular disease or retinal thrombosis or deep venous thrombosis, while undergoing therapy with SAYANAJECT should not be readministered the drug. Women with a prior history of thromboembolic disorders have not been studied in clinical trials and no information is available that would support the safety of SAYANAJECT use in this population.
Anaphylaxis and Anaphylactoid Reaction
If an anaphylactic reaction occurs appropriate therapy should be instituted. Serious anaphylactic reactions require emergency medical treatment.
Ocular Disorders
Medication should not be re-administered pending examination if there is a sudden partial or complete loss of vision or if there is a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, medication should not be re-administered.
Precautions
Weight Changes
Weight changes are common but unpredictable. In the phase 3 studies body weight was followed over 12 months. Half (50%) of women remained within 2.2 Kg of their initial body weight. 12% of women lost more than 2.2 Kg, and 38% of women gained more than 2.3 Kg.
Fluid Retention
There is evidence that progestogens may cause some degree of fluid retention, and as a result, caution should be exercised in treating any patient with a pre-existing medical condition that might be adversely affected by fluid retention.
Return of Ovulation
Following a single dose of DMPA subcutaneous injection, the cumulative rate of return to ovulation as measured by plasma progesterone was 97.4% (38/39 patients) by one year after administration. After the 14-week therapeutic window, the earliest return to ovulation was one week, and the median time to ovulation was 30 weeks. Women should be counseled that there is a potential for delay in return to ovulation following use of the method, regardless of the duration of use. It is recognised, however, that amenorrhoea and/or irregular menstruation upon discontinuation of hormonal contraception may be due to an underlying disorder associated with menstrual irregularity especially polycystic ovarian syndrome.
Psychiatric Disorders
Patients with a history of treatment for clinical depression should be carefully monitored while receiving SAYANAJECT.
Protection Against Sexually Transmitted Diseases
Patients should be counselled that SAYANAJECT does not protect against HIV infection (AIDS) or other sexually transmitted diseases.
Carbohydrate/Metabolism
Some patients receiving progestogens may exhibit a decrease in glucose tolerance. Diabetic patients should be carefully observed while receiving such therapy.
Liver Function
If jaundice develops in any woman receiving SAYANAJECT , consideration should be given to not re-administer the medication. (see section 4.3)
Hypertension and Lipid disorders
Limited evidence suggests that there is a small increased risk of cardiovascular events among women with hypertension or with lipid disorders who used progestogen-only injectables. If hypertension occurs under SAYANAJECT treatment and/or the increase in hypertension cannot adequately be controlled by antihypertensive medication, treatment with SAYANAJECT should be stopped. Additional risk factors for arterial thrombotic disorders include: Hypertension, smoking, age, lipid disorders, migraine, obesity, positive family history, cardiac valve disorders, atrial fibrillation.
SAYANAJECT should be used cautiously in patients with one or more of these risk factors.
Other conditions
The following conditions have been reported both during pregnancy and during sex steroid use, but an association with the use of progestagens has not been established: jaundice and/or pruritus related to cholestasis; gallstone formation; porphyria; systemic lupus erythematosus; hemolytic uraemic syndrome; Sydenham's chorea; herpes gestationis; otosclerosis-related hearing loss.
If any of the conditions/risk factors mentioned is present, the benefits of SAYANAJECT use should be weighed against the possible risks for each individual woman and discussed with the woman before she decides to start using it. In the event of aggravation, exacerbation or first appearance of any of these conditions or risk factors, the woman should contact her physician. The physician should then decide on whether SAYANAJECT use should be discontinued.
Laboratory Tests
The pathologist should be advised of progestogen therapy when relevant specimens are submitted. The physician should be informed that certain endocrine and liver function tests, and blood components might be affected by progestogen therapy:
a) Plasma/urinary steroids are decreased (e.g. progesterone, estradiol, pregnanediol, testosterone, cortisol)
b) Plasma and urinary gonadotropin levels are decreased (e.g., LH, FSH).
c) Sex-hormone-binding-globulin (SHBG) concentrations are decreased.
Important information about excipients
As this product contains methylparahydroxbenzoate (E218) and propylparahydroxbenzoate (E216), it may cause allergic reactions (possibly delayed) and exceptionally, bronchospasm. This medicinal product contains less than 1 mmol sodium (23 mg) per 104 mg/0.65 ml, i.e. essentially ‘sodium-free’.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed with SAYANAJECT.
Interactions with other medical treatments (including oral anticoagulants) have rarely been reported, but causality has not been determined. The possibility of interactions should be borne in mind in patients receiving concurrent treatment with other drugs.
MPA is metabolized in vitro primarily by hydroxylation via the CYP3A4. Specific drug-drug interaction studies evaluating the clinical effects with CYP3A4 inducers or inhibitors on MPA have not been conducted and therefore the clinical effects of CYP3A4 inducers or inhibitors are unknown.
4.6 Fertility, pregnancy and lactation
Fertility
SAYANAJECT is indicated for the prevention of pregnancy.
Women may experience a delay in return to fertility (conception) following discontinuation of SAYANAJECT (see section 4.4).
Pregnancy
SAYANAJECT is contraindicated in women who are pregnant. Some reports suggest an association between intrauterine exposure to progestational drugs in the first trimester of pregnancy and genital abnormalities in male and female fetuses. If SAYANAJECT is used during pregnancy, or if the patient becomes pregnant while using this drug, the patient should be warned of the potential hazard to the fetus.
One study found that infants from unintentional pregnancies that occurred 1 to 2 months after injection of medroxyprogesterone acetate Injection 150 mg IM were at an increased risk of low birth weight; this, in turn, has been associated with an increased risk of neonatal death. However, the overall risk of this is very low because pregnancies while on medroxyprogesterone acetate Injection 150 mg IM are uncommon.
Children exposed to MPA in utero and followed to adolescence showed no evidence of any adverse effects on their health including their physical, intellectual, sexual or social development.
Lactation
Low detectable amounts of drug have been identified in the milk of mothers receiving MPA. In nursing mothers treated with medroxyprogesterone acetate injection 150 mg IM, milk composition, quality, and amount are not adversely affected. Neonates and infants exposed to MPA from breast milk have been studied for developmental and behavioural effects through puberty. No adverse effects have been noted. However, due to limitations of the data regarding the effects of MPA in breastfed infants less than six weeks old, SAYANAJECT should be given no sooner than six weeks post partum when the infant’s enzyme system is more developed.
4.7 Effects on ability to drive and use machines
SAYANAJECT has no influence on the ability to drive and use machines.
4.8 Undesirable effects
Events from clinical trials:
The table below provides a listing of adverse drug reactions with frequency based on allcausality data from clinical studies that enrolled 2053 women who received DMPA-SC for contraception. The most frequently (>5%) reported adverse drug reactions were headache (8.9%), metrorrhagia (7.1%), weight increased (6.9%), amenorrhoea (6.3%) and injection site reactions (any type, 6.1%).
Adverse reactions are listed according to the following categories. These are as follows:
Very Common (>1/10)
Common (>1/100 to <1/10)
Uncommon (>1/1,000 to <1/100)
Rare (>1/10,000 to <1/1,000)
Frequency not known (cannot be estimated from the available data)
Events from post-marketing surveillance:
In addition, adverse events of medical significance obtained from post-marketing data with the use of injectable DMPA (IM or SC) are also included in the list below:
|
System organ class |
Very Common |
Common |
Uncommon |
Rare |
Not known |
|
Neoplasms benign, malignant and unspecified (including cysts and polyps) |
Breast cancer (see section 4.4) | ||||
|
Immune system disorders |
Drug hypersensitivity (see section 4.4) |
Anaphylactic reaction, Anaphylactoid reaction, Angioedema (see section 4.4) | |||
|
Metabolism and nutrition disorders |
Fluid retention (see section 4.4), Increased appetite, Decreased appetite | ||||
|
Pyschiatric disorders |
Depression, Insomnia, Anxiety, Affective disorder, Irritability, Libido decreased |
Nervousness, Emotional disorder, Anorgasmia | |||
|
Nervous system disorders |
Dizziness, Headache |
Migraine, Somnolence |
Seizure | ||
|
Ear and labyrinth disorders |
Vertigo | ||||
|
Cardiac disorders |
Tachycardia | ||||
|
Vascular disorders |
Hypertension (see section 4.4), Varicose vein, Hot flush |
Pulmonary embolism, Embolism and thrombosis, (see section 4.4), Thrombophlebiti | |||
|
Gastrointestinal disorders |
Abdominal pain, Nausea |
Abdominal distension | |||
|
Hepatobiliary disorders |
Jaundice, Hepati function abnorm; (see section 4.4 | ||||
|
Skin and subcutaneous tissue disorders |
Acne |
Alopecia, Hirsutism, Dermatitis, |
Lipodys trophy acquired |
Skin striae |
|
System organ class |
Very Common |
Common |
Uncommon |
Rare |
Not known |
|
Ecchymosis, Chloasma, Rash, Pruritus, Urticaria | |||||
|
Musculoskeletal and connective tissue disorders |
Back pain, Pain in extremity |
Arthralgia, Muscle spasms |
Osteoporosis, Osteoporotic fractures | ||
|
Reproductive System & Breast Disorders |
Menometrorrhagia, Metrorrhagia, Menorrhagia (see section 4.4), Dysmenorrhoea, Amenorrhoea, Vaginitis, Breast pain |
Ovarian cyst, Uterine haemorrhage (irregular, increase, decrease), Vaginal discharge, Dyspareunia, Galactorrhoea, Pelvic pain, Vulvovaginal dryness, Premenstrual syndrome, Breast tenderness, Breast enlargement | |||
|
General disorders and administration site conditions |
Fatigue, Injection site reaction, Injection site persistent atrophy/Indentation /dimpling, Injection site nodule/lump, Injection site pain/tenderness |
Pyrexia |
Astheni a | ||
|
Investigations |
Weight increased (see section 4.4), Smear cervix abnormal |
Bone density decreased (see section 4.4), Glucose tolerance decreased (see section 4.4), Hepatic enzyme abnormal |
Weight decrease d (see section 4.4) |
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product.
Healthcare professionals are asked to report any suspected adverse reactions via Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard.
4.9 Overdose
No positive action is required other than cessation of therapy.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: progestogens, ATC Code: G03AC06
MPA is an analogue of 17 a-hydroxyprogesterone with anti-estrogenic, anti-androgenic and antigonadotrophic effects.
DMPA subcutaneous injection inhibits the secretion of gonadotropins which, in turn, prevents follicular maturation and ovulation and causes thickening of cervical mucus which inhibits sperm entry into the uterus. T hese actions produce its contraceptive effect.
BMD Changes in Adult Women
A study comparing changes in BMD in women using DMPA subcutaneous injection with women using medroxyprogesterone acetate injection (150 mg IM) showed no significant differences in BMD loss between the two groups after two years of treatment. Mean percent changes in BMD in the DMPA subcutaneous injection group are listed in Table 1.
Table 1. Mean Percent Change from Baseline in BMD in Women Using DMPA
subcutaneous
injection by Skeletal Site
|
Time on Treatment |
Lumbar Spine |
Total |
Hip |
Femoral Neck | ||
|
N |
Mean % Change (95% CI) |
N |
Mean % Change (95% CI) |
N |
Mean % Change (95% CI) | |
|
1 year |
166 |
-2.7 (-3.1 to -2.3) |
166 |
-1.7 (-2.1 to -1.3) |
166 |
-1.9 (-2.5 to -1.4) |
|
2 year |
106 |
- 4.1 (-4.6 to -3.5) |
106 |
-3.5 (-4.2 to -2.7) |
106 |
-3.5 (-4.3 to -2.6) |
In another controlled, clinical study adult women using medroxyprogesterone acetate injection (150 mg IM) for up to 5 years showed spine and hip mean BMD decreases of 5-6%, compared to no significant change in BMD in the control group. The decline in BMD was more pronounced during the first two years of use, with smaller declines in subsequent years. Mean changes in lumbar spine BMD of -2.86%, -4.11%, -4.89%, -4.93% and -5.38% after 1, 2, 3, 4 and 5 years, respectively, were observed. Mean decreases in BMD of the total hip and femoral neck were similar. Please refer to Table 2 below for further details.
After stopping use of medroxyprogesterone acetate injection (150 mg IM), BMD increased towards baseline values during the post-therapy period. A longer duration of treatment was associated with a slower rate of BMD recovery.
Table 2. Mean Percent Change from Baseline in BMD in Adults by Skeletal Site and Cohort after 5 Years of Therapy with Medroxyprogesterone acetate 150 mg IM and after 2 Years Post-Therapy or 7 Years of Observation (Control)
|
Time in Study |
Spine |
Total Hip |
Femoral Neck | |||
|
Medroxyprog esterone acetate |
Control |
Medroxyprog esterone acetate |
Control |
Medroxyprog esterone acetate |
Control | |
|
5 years1 |
n=33 -5.38% |
n=105 0.43% |
n=21 -5.16% |
n=65 0.19% |
n=34 -6.12% |
n=106 -0.27% |
|
7 years2 |
n=12 -3.13% |
n=60 0.53% |
n=7 -1.34% |
n=39 0.94% |
n=13 -5.38% |
n=63 -0.11% |
that, based on mean values, lumbar spine BMD recovered to baseline levels approximately 1 year after treatment was discontinued and that hip BMD recovered to baseline levels approximately 3 years after treatment was discontinued. However, it is important to note that a large number of subjects discontinued from the study, therefore these results are based on a small number of subjects (n=71 at 60 weeks and n=25 at 240 weeks after treatment discontinuation). In contrast, a non-comparable cohort of unmatched, untreated subjects, with different baseline bone parameters from the DMPA users, showed mean BMD increases at 240 weeks of 6.4%, 1.7% and 1.9% for lumbar spine, total hip and femoral neck, respectively.
5.2 Pharmacokinetic properties
The pharmacokinetic parameters of MPA following a single SC injection of DMPA are shown in Table 1.
Table 1. Pharmacokinetic Parameters of MPA
After a Single SC Injection of DMPA in ^ Healthy Women (n = 42)
|
Cmax |
T Amax |
C91 (min) |
AUC0-91 |
AUC0- |
t/ | |
|
(ng/ml) |
(day) |
(ng/ml) |
(ngday/ml) |
(ngday/ml) |
(day) | |
|
Mean |
1.56 |
8.8 |
0.402 |
66.98 |
92.84 |
43 |
|
Min |
0.53 |
2.0 |
0.133 |
20.63 |
31.36 |
16 |
|
Max |
3.08 |
80.0 |
0.733 |
139.79 |
162.29 |
114 |
Cmax = peak serum concentration; Tmax = time when Cmax is observed; AUC0-91 = area under the concentration-time curve over 91 days; t/ = terminal half-life; 1 nanogram
3
= 10 picogram.
General Characteristics
Absorption
MPA absorption from the SC injection site to achieve therapeutic levels is relatively prompt. The mean Tmax attained approximately one week after injection. The peak MPA concentrations (Cmax) generally range from 0.5 to 3.0 ng/ml with a mean Cmax of 1.5 ng/ml after a single SC injection.
Effect of Injection Site
DMPA was administered subcutaneously into the anterior thigh or the abdomen to evaluate effects on MPA concentration-time profile. MPA trough concentrations (Cmin; Day 91) were similar for the two injection locations, suggesting that injection site does not negatively affect the contraceptive efficacy.
Distribution
Plasma protein binding of MPA averages 86%. MPA binding occurs primarily to serum albumin; no binding of MPA occurs with SHBG.
Biotransformation
MPA is extensively metabolized in the liver by P450 enzymes. Its metabolism primarily involves ring A and/or side-chain reduction, loss of the acetyl group, hydroxylation in the 2-, 6-, and 21-positions or a combination of these positions, resulting in more than 10 metabolites.
Elimination
Residual MPA concentrations at the end of the dosing interval (3 months) of DMPA subcutaneous
injection are generally below 0.5 ng/ml, consistent with its apparent terminal half-life of ~40 days after SC administration. Most MPA metabolites are excreted in the urine as glucuronide conjugates with only small amounts excreted as sulfates.
Linearity/non-linearity
Based on single-dose data, there was no evidence of non-linearity over the dose range of 50 to 150 mg after SC administration. The relationship between the AUC or the Cmin and the SC dose of MPA appeared to be linear. The mean Cmax did not change substantially with increasing dose
Race
There were no apparent differences in the pharmacokinetics and/or dynamics of MPA after SC administration of DMPA among women of all ethnic backgrounds studied. The pharmacokinetics/dynamics of MPA has been evaluated in Asian women in a separate study.
Effect of Body Weight
No dosage adjustment of SAYANAJECT is necessary based on body weight. The effect of body weight on the pharmacokinetics of MPA was assessed in a subset of women (n = 42, body mass index [BMI] ranged from 18.2 to 46.0 kg/m2). The AUC0. 9i values for MPA were 68.5, 74.8, and 61.8 ng -day/ml in women with BMI categories of < 25 kg/m2, >25 to <30 kg/m2, and >30 kg/m2, respectively. The mean MPA Cmax was 1.65 ng/ml in women with BMI < 25 kg/m2, 1.76 ng/ml in women with BMI >25 to <30 kg/m2, and 1.40 ng/ml in women with BMI > 30 kg/m2, respectively. The range of MPA trough (Cmin) concentrations and the half-lives were comparable for the 3 BMI groups.
Pharmacokinetic/Pharmacodynamic Relationship(s)
From a pharmacodynamic perspective, the duration of ovulation suppression depends upon maintaining therapeutic MPA concentrations throughout the 13 week dosing interval.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity and carcinogenic potential. Medroxyprogestrone acetate has been shown to have adverse effects on reproduction in animals and is contraindicated for use during pregnancy.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Macrogol 3350
Methyl parahydroxybenzoate (E 218)
Propyl parahydroxybenzoate (E 216)
Sodium Chloride Polysorbate 80
Monobasic Sodium Phosphate Monohydrate Disodium Phosphate Dodecahydrate Methionine Povidone
Hydrochloric Acid and/or Sodium Hydroxide for pH adjustment Water for Injection
6.2 Incompatibilities
Not applicable
6.3 Shelf life
Unopened: 5 years
Once opened: use immediately, discard any unused portion
6.4 Special precautions for storage
Do not refrigerate or freeze
6.5 Nature and contents of container
SAYANAJECT suspension for injection is supplied in a single-dose container in the form of a pre-filled injector containing 0.65 ml. The injector comprises a linear low
density polyethylene laminate reservoir with a siliconized AISI Type 304 Stainless Steel 23 gauge ultra thin wall needle attached via a low density polyethylene port and valve.
6.6 Special precautions for disposal
For single use only.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Instructions for Use and Handling
Getting ready
• Ensure that the medicine is at room temperature.
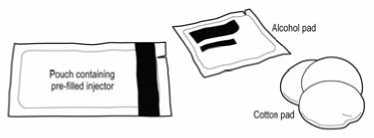
• Make sure the following components are available:
- One sealed foil pouch containing SAYANAJECT in the pre-filled injector
- An alcohol pad
- A clean cotton pad
(The alcohol pad and the cotton pad are not supplied with the SAYANAJECT product.)
Step 1: Choosing and preparing the injection area
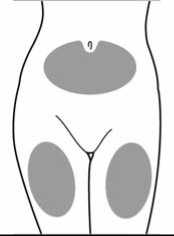
• Choose a suitable area for subcutaneous injection, either the abdomen or the front upper thigh. Avoid bony areas and the umbilicus (navel).
• Use an alcohol pad to wipe the skin in the injection area you have chosen. Allow the skin to dry.
Step 2: Preparing the injector
• When you are ready to give the injection, carefully tear open the foil pouch, and remove the injector. Do not remove the needle shield at this stage.
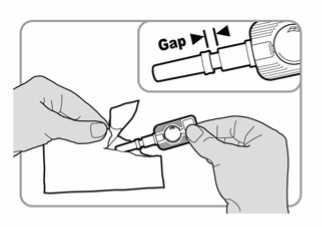
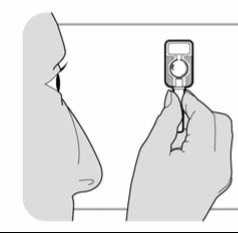
• Check the injector as follows:
- The needle shield should be in the position shown in the diagram. There should be a gap between the end of the needle shield and the port.
- If there is no gap, discard the injector and use a new one.
- If the needle shield has come off the needle, or is missing altogether, discard the injector and use a new one.
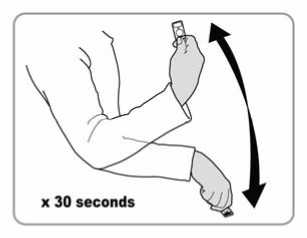
Step 3: Mixing the medicine
• Hold the injector firmly by the port. (See figure 1 for port location.)
• Shake the injector vigorously for 30 seconds to mix the medicine thoroughly. Do not bend it.
• If there is any delay between mixing the medicine and proceeding through the next steps, repeat the mixing procedure as above.
Check the injector. The liquid contents should appear white to off-white and uniform. There should be no leakage from any part.
If any problems are observed, discard the injector and use a new one.
Step 4: Activating the injector
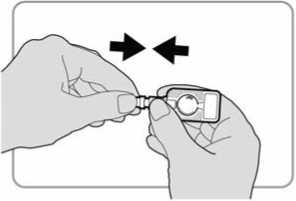
• Hold the injector firmly by the port with one hand. Take care not to squeeze the reservoir.
• Hold the needle shield with the other hand. You will see a gap between the port and the end of the needle shield.
Push the needle shield towards the port. Continue to push firmly until you close the gap between the needle shield and the port. The injector is now activated.
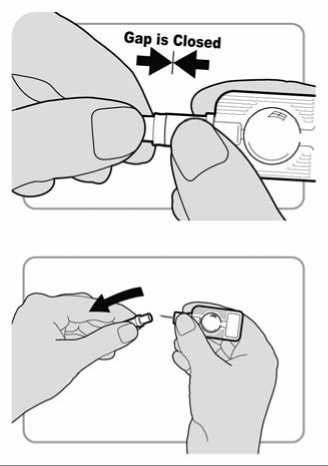
Continue to hold the injector firmly by the port.
Pull the needle shield away to remove it from the needle.
7
MARKETING AUTHORISATION HOLDER
Pfizer Limited Ramsgate Road Sandwich Kent
CT13 9NJ United Kingdom
8
9
MARKETING AUTHORISATION NUMBER(S)
PL 00057/1498
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
23/12/2014
DATE OF REVISION OF THE TEXT
28/07/2016
The treatment group consisted of women who received medroxyprogesterone acetate injection (150 mg IM) for 5 years and the control group consisted of women who did not use hormonal contraception for this time period.
The treatment group consisted of women who received medroxyprogesterone acetate Injection (150 mg IM) for 5 years and were then followed up for 2 years post-use and the control group consisted of women who did not use hormonal contraceptive for 7 years.
BMD Changes in Adolescent Females (12-18 years)
Results from an open-label, non-randomised, clinical study of medroxyprogesterone acetate Injection (150 mg IM every 12 weeks for up to 240 weeks (4.6 years), followed by posttreatment measurements) in adolescent females (12-18 years) also showed that medroxyprogesterone acetate IM use was associated with a significant decline in BMD from baseline. Among subjects who received > 4 injections/60-week period, the mean decrease in lumbar spine BMD was - 2.1 % after 240 weeks (4.6 years); mean decreases for the total hip and femoral neck were -6.4 % and -5.4 %, respectively. Post-treatment follow-up showed