Scopoderm 1.5 Mg Patch
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Scopoderm 1.5mg Patch
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each patch contains 1.5mg hyoscine U.S.P.
For the full list of excipients, see section 6.1
3 PHARMACEUTICAL FORM
Transdermal Patch
It is a flat, round reservoir patch approximately 1.8 cm in diameter. One side of the patch is tan; the other side is silver and is placed on an oversized clear hexagonal film. Each patch is a flat system of laminates, sealed around the edge, containing a clear oily filling. Each system has a contact surface area measuring 2.5cm and hyoscine content of 1.5mg. The average amount of hyoscine absorbed from each system in 72 hours is 1mg.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
For the prevention of travel sickness symptoms e.g. nausea, vomiting and vertigo.
4.2 Posology and method of administration
Route of Administration Transdermal
Posology
Adults
To achieve the optimum protective effect, Scopoderm Patch should be applied about 5-6 hours before embarking on a journey (or on the evening before the journey). The system should be placed onto a clean, dry, hairless area of skin behind the ear, taking care to avoid any cuts or irritation (see "Instructions for use"). Application of one Scopoderm Patch is quite sufficient to ensure protection for up to 72 hours. Should protection be required for longer periods of time, the scopoderm Patch must be removed after 72 hours and a fresh system applied behind the other. (No more than one system should be used at a time). Conversely, if protection is only required for a shorter period of time, the system should be removed at the end of the journey.
To prevent traces of active substance from entering the eyes - which might lead to slight temporary blurring of vision and to dilatation of the pupils (sometimes in one eye only) - patients should wash their hands thoroughly after handling the system. In addition, after removal of the system, the site of application should also be washed. These precautions are necessary to minimise any chance of hyoscine accidentally being transferred to the eyes (see side-effects).
Limited contact with water (i.e. during bathing or swimming), should not affect the system, although it should be kept as dry as possible.
If the Scopoderm Patch, which normally adheres well to the skin, becomes accidentally detached, it should be replaced by a fresh system.
Use in Elderly
Scopoderm Patch may be used in the elderly (see dosage recommendations for adults) although the elderly may be more prone to suffer from the side-effects of hyoscine (see precautions).
Paediatric population
Scopoderm Patch can be used in children age 10 years or over (see dosage recommendations for adults). The safety and efficacy of Scopoderm Patch for children younger than 10 years have not been established.
Instructions for use
Tear open the sachet at the top and take out the flesh-colored system complete with its transparent hexagonal protective foil (Fig. 1).
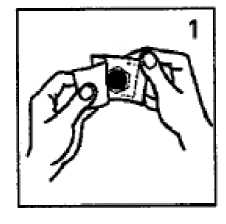
Holding the system only by its edge - and taking care if possible not to touch the silvery adhesive side (Fig. 2) - peel off the hexagonal foil.
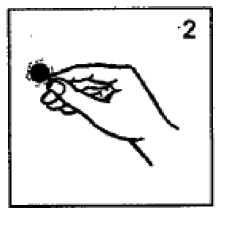
Press the system (silvery adhesive side downwards) firmly on to a clean, dry, hairless area of skin behind the ear (Fig. 3).
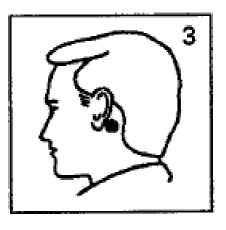
Once the system has been affixed, it should not be touched again while it is being worn, since pressure exerted on it might possibly cause scopolamine to ooze out at the edge.
After the system has been either applied or removed, the hand (and, after its removal, also the site of application) should be thoroughly washed.
4.3 Contraindications
Scopoderm Patch is contra-indicated in patients with glaucoma or with a history of the condition, and in patients with known hypersensitivity to hyoscine or to any of the excipients listed in section 6.1.
4.4 Special warnings and precautions for use
Scopoderm Patch should be used with caution in patients with pyloric stenosis or those who have difficulty in passing water owing to an impeded flow of urine (e.g. in diseases of the prostate), as well as in patients with intestinal obstruction.
Patients should not consume alcohol whilst using Scopoderm Patch.
Scopoderm Patch should also be used with caution in elderly patients, and in patients with impaired hepatic or renal function.
In patients whose case history indicates that there might be raised intra-ocular pressure (pressure pain, blurred vision, glaucomatous halo), Scopoderm Patch should only be employed after an ophthalmological examination.
In rare cases, confusional states and visual hallucinations may occur. In such cases, Scopoderm Patch should be removed immediately. If severe symptoms persist in a severe form, appropriate therapeutic measures should be taken e.g. administration of physostigmine, 1-4 mg (in children 0.5 mg), slowly IV (intravenous) to be repeated if necessary (see overdose section).
Idiosyncratic reactions may occur with ordinary therapeutic doses of hyoscine.
In isolated cases an increase in seizure frequency in epileptic patients has been reported.
Care should be taken after removal of the system as side-effects may persist for up to 24 hours or longer.
Due the presence of aluminium in one of the layers of the system, the patch should be removed before medical scans.
4.5 Interaction with other medicinal products and other forms of interaction
Scopoderm Patch should be used with caution in patients being treated with drugs that act on the central nervous system or drugs with anticholinergic properties e.g. other belladonna alkaloids, antihistamines, tricyclic antidepressants (such as amitriptyline and imipramine), amantadine, quinidine.
Patients should refrain from consuming alcohol during use of Scopoderm Patch.
4.6 Pregnancy and lactation
Teratogenic studies have been performed in pregnant rats and rabbits with hyoscine administered by daily intravenous injection. No adverse effects were noted in rats. In rabbits, the drug had a marginal embryotoxic effect at a high dose (at drug plasma levels approximately 100 times those observed in humans using Scopoderm Patch).
Scopoderm Patch should only be used during pregnancy if the expected benefits to the mother outweigh the potential risks to the foetus.
As scopolamine is excreted in human milk, although only in trace amounts, caution should be exercised when Scopoderm Patch is administered to a nursing woman.
4.7 Effects on ability to drive and use machines
Scopoderm may cause drowsiness, dizziness, confusion or visual disturbance in certain individuals. Patients using the system must not drive, operate machinery, pilot an aircraft, dive or engage in any other activities in which such symptoms could be dangerous (see side-effects).
4.8 Undesirable effects
Adverse reactions are listed below by system organ class and frequency.
Frequencies are defined as: very common (> 1/10); common (> 1/100 to < 1/10); uncommon (> 1/1,000 to < 1/100); rare (> 1/10,000 to < 1/1,000); very rare (< 1/10,000), or not known (can not to be estimated from available data). Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness.
Psychiatric disorders
Rare: disorientation, confusion and hallucinations.
Nervous system disorders
Very common: somnolence, dizziness.
Rare: memory impairment, disturbance in attention, restlessness, disorientation, confusion and visual hallucinations. (see precautions).
Eye disorders
Very common: disturbances of visual accommodation (cycloplegia) including blurred vision, myopia and mydriasis (sometimes unilateral)
Common eyelid irritation
Very rare: pupillary dilatation may precipitate acute glaucoma, particularly narrow angle glaucoma (see Contra-Indications).
Gastrointestinal disorders
Very common: dryness of the mouth.
Skin and subcutaneous tissue disorders Common: skin irritation Very rare: rash generalised
Renal and urinary disorders Rare: urinary retention
Side-effects after removal of Scopoderm Patch
After discontinuation of treatment, in rare cases - usually after several days of use - symptoms such as dizziness, nausea, vomiting, headache, and disturbances of balance have been reported. In such cases, patients should not drive or engage in other activities requiring concentration (see warnings).
Reporting of suspected adverse reactions
Reporting of suspect adverse reactions is an important way to continuously monitor the benefit/risk balance of the medicinal product in the real conditions of use. Healthcare professionals are asked to report any suspected adverse reaction via the Yellow Card Scheme, www.mhra.gov.uk/yellowcard.
4.9 Overdose
Symptoms
Initially, restlessness, excitation and confusion may be observed. In response to higher doses, delirium, hallucinations and convulsions set in. At very high doses, coma and respiratory paralysis may occur.
Treatment
If symptoms of overdosage occur, the system(s) should be removed immediately as some overdose symptoms may persist for up to 24 hours or longer even after patch removal. Physostigmine is the most effective antidote. Depending on the severity of poisoning, physostigmine should be given by slow intravenous injection in doses of 1-4mg (0.5mg in children). Repeated injections may be necessary since physostigmine is rapidly metabolised. Diazepam may be used to counter excitation and convulsions although at higher doses it may cause respiratory depression. In severe cases, artificial respiration may be necessary. If hyperthermia occurs, immediate action should be taken to dissipate heat.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Antiemetics and antinauseants, ATC code: A04AD01
The transdermal therapeutic system (TTS) is a novel form of drug delivery designed to achieve a continuous release of hyoscine through the intact skin to the systemic circulation for up to 72 hours.
Hyoscine is a naturally occurring belladonna alkaloid and has anticholinergic properties. It acts as a competitive antagonist to acetylchloline and other parasympathomimetic agents. Its mechanism of action in the central nervous system in preventing motion sickness has yet to be elucidated. The ability of hyosine to prevent nausea and vomiting due to motion sickness may be related to inhibition of cholinergic impulse conduction from the vestibular nucleus to the higher centers of the central nervous system, as well as from the reticular formation to the vomiting centre. Hyoscine produces classical symptoms of parasympathetic blockade.
5.2 Pharmacokinetic properties
Following Scopoderm Patch administration, measurement of the urinary excretion has shown the equilibrium between absorption and elimination to be reached within about 6 hours. Steady plasma concentrations of hyoscine in the range of 0.17-0.33nmol/litre are produced. Provided the system is not removed, this equilibrium is maintained and plasma hyoscine levels are within this therapeutic range for up to 72 hours.
Little data about the distribution of scopolamine is available; however the drug distributes well and reaches the central nervous system. Scopolamine seems to be bound to plasma proteins in a reversible manner.
The metabolism of scopolamine has not been fully characterised. The drug appears to be metabolised in the liver (glucoronide or sulfate conjugation).
After removal of Scopoderm Patch, the plasma concentration diminishes slowly to approximately one third over the following 24 hours because hyoscine in the skin continues to enter the blood stream.
Scopolamine is excreted in urine. The urinary excretion rate of free and total (free plus conjugated) scopolamine was about 0.7 and 3.8 micrograms/hour, respectively after the application of a single transdermal scopolamine system. Less than 10% of the total dose is excreted in urine as unchanged drug and its metabolites over 108 hours.
Following a single application of two transdermal scopolamine systems, the average elimination half-life of the drug (free scopolamine) was 9.5 hours.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of repeated dose toxicity, skin irritation, genotoxicity, carcinogenic potential and toxicity to reproduction. A marginal embryotoxic effect was seen in rabbits with scopolamine hydrobromide administered by daily intravenous injection at doses that were approximately 100 times the level achieved with transdermal systems. No adverse effects were recorded in reprotoxicity studies following IV administration in rats.
6.1 List of excipients
Drug Reservoir
Light mineral oil Polyisobutylene (1.200.000)
Polyisobutylene (35.000)
Backing Film
Pigmented MDPE/AL/PET/HS Film (vapour coated aluminised polyester with outer coating of pigmented medium density polyethylene (MDPE) and a heat sealable inner coating. Thickness 0.0686 mm.
Release Controlling Membrane
Polypropylene Film. Thickness 0.0254 mm.
Adhesive (to skin)
Light mineral oil Polyisobutylene (1.200.000)
Polyisobutylene (35.000)
Release Liner (discarded before use)
Silicone/Polyester Film Thickness 0.0762 mm
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
36 months
6.4 Special precautions for storage
Do not store above 25oC. Do not freeze.
6.5 Nature and contents of container
Scopoderm - Individually packed into sealed paper laminated aluminium foil pouches. Outer cardboard carton containing two patches.
6.6 Special precautions for disposal and other handling
The transdermal patch should be folded in half (sticky side inwards) before being discarded.
Patients should wash their hands thoroughly after handling the system. In addition, after removal of the system, the site of application should also be washed. These precautions are necessary to minimise any chance of hyoscine accidentally being transferred to the eyes (see side-effects).
7 MARKETING AUTHORISATION HOLDER
Novartis Consumer Health UK Limited
Trading as: Novartis Consumer Health Park View,
Riverside Way,
Watchmoor Park,
Camberley,
Surrey,
GU15 3YL,
UK.
8 MARKETING AUTHORISATION NUMBER
PL 00030/0211
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
16 March 2004 / 02 March 2009
10 DATE OF REVISION OF THE TEXT
19/03/2014