Scopoderm Tts Patches
PACKAGE LEAFLET: INFORMATION FOR THE USER
Scopoderm® TTS Patches
Hyoscine 1.5mg
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Scopoderm TTS Patches are and what they are used for
2. Before you use Scopoderm TTS Patches
3. How to use Scopoderm TTS Patches
4. Possible side effects
5. How to store Scopoderm TTS Patches
6. Further information
1. What Scopoderm TTS Patches are and what they are used for
Scopoderm TTS Patches contain the active substance hyoscine which belongs to the group of medicines called anti-emetics or anti-sickness medicines. They are used to prevent the symptoms of motion sickness such as nausea (feeling sick), vomiting (being sick) and vertigo (loss of balance), which can occur when travelling by boat train or car.
2. Before you use Scopoderm TTS Patches
Do not use Scopoderm TTS Patches if you:
• have glaucoma or a history of glaucoma (pressure behind the eye)
• are allergic (hypersensitive) to hyoscine or any of the other ingredients in the patch (see Section 6).
Talk to your doctor or pharmacist before using Scopoderm TTS Patches if you:
• suffer from pyloric stenosis (a condition which affects your stomach)
• have problems urinating due to a bladder obstruction
• have a blockage of your intestines
• are an elderly patient or have any metabolic, liver or kidney disease
• suffer from epilepsy (increased number of fits have been reported)
• have had pain in the eyes, blurred vision, or see rainbow-coloured halos around lights (Scopoderm TTS should only be used after an eye examination by a doctor).
Under these circumstances, Scopoderm TTS Patches may be unsuitable for you.
Care should be taken after removal of the patch as side effects may persist for up to 24 hours or longer.
Remove the patch before a medical scan as aluminium is present in one of the patch layers.
Scopoderm TTS patches are not recommended for use in children under 10 years of age.
Taking other medicines with Scopoderm TTS Patches
Please tell your doctor or pharmacist if you are taking, or have recently taken, any other medicines, including those obtained without a prescription.
In particular, tell your doctor if you are taking any of the following medicines, because Scopoderm may interact with them:
• alcohol
• other drugs acting on the brain
• antiallergic medicines
• antidepressants
• antiparkinsons and antivirals
• antiarrhythmics
• other travel sickness medicines.
Using Scopoderm TTS Patches with food and drink
Do not drink alcohol whilst using the patches or until the effects of the patches have worn off (this may last up to 24 hours or longer from the time you have removed it).
Pregnancy and breast-feeding
Do not use Scopoderm TTS Patches during pregnancy and breast-feeding unless your doctor advises you to.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Scopoderm TTS Patches may make you feel drowsy, confused or dizzy and may affect your vision. Do not drive, operate any machinery or perform any activity that requires concentration. Care should be taken after removal of the patch as these effects may persist for up to 24 hours or longer.
3. How to use Scopoderm TTS Patches
Always use the patches exactly as described in this leaflet or as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Adults
One patch should be applied about 5-6 hours before leaving on your journey (or the evening before) to a clean, dry, hairless area of intact skin behind the ear.
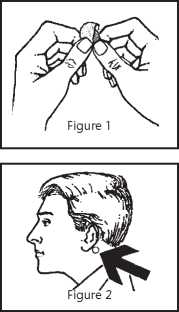
1. Remove the patch from the sachet. Peel off the clear, hexagonal plastic backing, holding the patch by its edge so as not to touch the sticky side (Figure 1).
2. Press the silver coloured sticky side of the patch firmly on to a clean, dry, hairless area of skin behind the ear (Figure 2). Avoid areas of skin that are cut or irritated in any way.
3. Wash your hands thoroughly after applying the patch. Do not touch the patch once it is in place as you may get the active ingredient on your fingers. If you do touch the patch, wash your hands immediately. Do not get the active ingredient near your eyes. If you get hyoscine on your fingers then touch your eyes, your vision may be temporarily affected.
4.Once your journey is over, take the patch off and dispose of it carefully.
5.When you take a patch off wash your hands and also the area of skin where the patch has been.
Each patch will last for up to 3 days. If you are travelling for more than three days, take the patch off after 3 days and apply a fresh patch behind the other ear. You can swim, bathe or shower with little risk of the patch coming off, provided that you have applied the patch properly. However, it is best not to stay in the water too long. If the patch accidentally comes off, dispose of it carefully and put on a new patch.
Elderly
Scopoderm TTS Patches may be used in the elderly (see dosage for adults) although they may be more prone to suffer from the side-effects of hyoscine (see Section 4: Possible Side Effects).
Children
Scopoderm TTS patches can be used in children aged 10 years or over (see dosage for adults).Do not exceed the recommended dose.
The use of these patches is not recommended in younger children. See your doctor or pharmacist for alternative medicines.
If you use more Scopoderm TTS Patches than you should
If you accidentally use too many patches, you may feel restless, excited or confused. In cases of higher overdose, you may become disorientated, hallucinate or have fits. In severe cases of overdose, coma and breathing difficulties may occur. Remove the patch /patches immediately, and tell your doctor or go to your nearest casualty department immediately. Take any remaining patches with you.
If you forget to use Scopoderm TTS Patches
If you forget to put a patch on, apply it as soon as you remember. Do not apply two patches at once to make up for the one that you forgot.
If you stop using Scopoderm TTS Patches
In rare cases - usually after several days of use - symptoms such as dizziness, nausea (feeling sick), vomiting (being sick), headache and disturbances of balance have been reported after discontinuation of the treatment.
These symptoms are more likely to occur if you have been wearing a patch for several days. If you are affected in this way, avoid any activities requiring concentration, e.g. driving, or operating machinery, until your symptoms have worn off and consult a doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Scopoderm TTS Patches can cause side effects, although not everybody gets them.
STOP using Scopoderm TTS and seek medical help immediately if you or your child has any of the following which may be signs of an allergic reaction:
• difficulty in breathing or swallowing
• swelling of the face, lips, tongue or throat
• severe itching of the skin, with a red rash or raised bumps
Some side effects could be serious which are very rare (may affect 1 in every 10,000 people)
• Changes in vision with increased pressure in the eye (possible signs of glaucoma)
If you experience this, tell your doctor straight away.
Some side effects are very common (may affect more than 1 in 10 people)
• dryness of the mouth.
• drowsiness, dizziness
• Frequently blurring of (near) vision and enlargement of the pupils (sometimes in one eye only),
• loss of ability to focus on close or far objects (visual accommodation)
Some side effects are common (may affect between 1 and 10 in every 100 people)
• irritation of the eyelids
• skin irritation
Some side effects are rare (may affect between 1 and 10 in every 10,000 people)
• difficulty in passing water (urinating)
• impairment of memory or concentration, restlessness, disorientation, confusion, or hallucinations.
Some side effects are very rare (may affect less than 1 in every 10,000 people)
• skin rash
If any of the side effects gets serious, or if you have any side effects not listed in this leaflet, please tell your doctor or pharmacist
5. How to store Scopoderm TTS Patches
Keep this medicine out of the reach and sight of children.
Do not store above 25°C.
Do not freeze the patches.
Do not remove from the protective pouch until you need it.
Do not use Scopoderm TTS Patches after the 'Expiry date' which is stated on the carton and pouch. The 'Expiry date' refers to the last day of that month.
The transdermal patch should be folded in half (sticky side inwards) before being discarded. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Further information
What Scopoderm TTS Patches contain
The active ingredient in each patch is hyoscine 1.5mg (the average amount of hyoscine absorbed from each patch in 72 hours is 1 mg).
The other ingredients are light mineral oil, polyisobutylene and polypropylene .
What Scopoderm TTS Patches look like and contents of the pack
Scopoderm TTS patches are flat, round patches approximately 1.8 cm in diameter. One side of the patch is tan; the other side is silver and is placed on an oversized clear hexagonal film.
Each patch is individually packed into a foil pouch. Each cardboard carton contains two or five pouches, not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Novartis Consumer Health UK Limited
Wimblehurst Road
Horsham
West Sussex
RH125AB
For any information about this medicinal product, please contact the Marketing Authorisation Holder. This medicinal product is authorised in the Member States of the EEA under the following names: This leaflet was last approved in January 2012.
GB_301042