Smofkabiven Emulsion For Infusion
PACKAGE LEAFLET: INFORMATION FOR THE USER
SmofKabiven emulsion for infusion
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What SmofKabiven is and what it is used for
2. Before you use SmofKabiven
3. How to use SmofKabiven
4. Possible side effects
5. How to store SmofKabiven
6. Further information
1. What SmofKabiven is and what it is used for
SmofKabiven is an emulsion for infusion given into your blood by a drip (intravenous infusion). The product contains amino acids (components used to build proteins), glucose (carbohydrates), lipids (fat) and salts (electrolytes) in a plastic bag.
A health care professional will give you SmofKabiven when other forms of feeding are not good enough or have not worked.
2. Before you use SmofKabiven
Do not use SmofKabiven:
- if you are allergic (hypersensitive) to any of the ingredients of SmofKabiven
- if you are allergic to fish or egg
- if you are allegic to peanuts or soya you should not use this product. SmofKabiven contains soya-bean
oil.
- if you have too much fat in the blood (hyperlipidemia)
- if you have serious liver disease
- if you have blood clotting problems (coagulation disorders)
- if your body has problems using amino acids
- if you have serious kidney disease without access to dialysis
- if you are in acute shock
- if you have too much sugar in your blood (hyperglycaemia) which is uncontrolled
- if you have high blood (serum) levels of the salts (electrolytes) included in SmofKabiven
- if you have fluid in the lungs (acute pulmonary oedema)
- if you have too much body fluid (hyperhydrated)
- if you have heart failure that is not treated
- if you have a defect in your blood clotting system (hemophagocytotic syndrome)
- if you are in an unstable condition, such as after serious trauma, uncontrolled diabetes, acute heart attack, stroke, blood clot, metabolic acidosis (a disturbance resulting in too much acid in the blood), serious infection (severe sepsis), coma and if you don’t have enough body fluid (hypotonic dehydration).
Take special care with SmofKabiven:
Tell your doctor if you have:
- kidney problems
- diabetes mellitus
- pancreatitis (inflammation of the pancreas)
- liver problems
- hypothyrodism (toxic goiter)
- sepsis (serious infection)
If you during the infusion get fever, rash, swelling, difficulty in breathing, chills, sweating, nausea or vomiting, tell the health care professional immediately because these symptoms might be caused by an allergic reaction or that you have been given too much of the medicine.
Your doctor may regularly need to check your blood for liver function tests and other values.
SmofKabiven is not meant for newborn babies or children younger than 2 years of age. At the moment, there is no experience of the use of SmofKabiven in children from 2 to 11 years old.
Taking other medicines:
Please tell your doctor if you have or recently have taken some medicines, even without prescription. Pregnancy and breast-feeding
Data from using SmofKabiven during pregnancy or breast-feeding is lacking. SmofKabiven should therefore be given to pregnant or breast-feeding womens only if the doctor find it necessary. The use of SmofKabiven may be considered during pregnancy and breastfeeding, as advised by your doctor.
Driving and using machines
Not relevant as the medicine is given at the hospital.
3. How to use SmofKabiven
Your doctor will decide on the dose for you individually depending on your body weight and function. SmofKabiven will be given to you by a health care professional.
If you get more SmofKabiven than you should
It is unlikely that you will receive too much medicine as SmofKabiven is given to you by a health care professional.
4. Possible side effects
Like all medicines, SmofKabiven can cause side effects, although not everybody gets them.
Common (occurs in more than 1 one in 100 patients): a slightly raised body temperature.
Uncommon (occurs in less than 1 in 100 patients but in more than 1 in 1000 of patients): high blood (plasma) levels of compounds from the liver, lack of appetite, nausea, vomiting, chills, dizziness and headache.
Rare (occurs in less than 1 in 1000 of patients but in more than 1 in 10000 patients): low or high blood pressure, difficulty in breathing, fast heart beat (tachycardia). Hypersensitivity reactions (that can give symptoms like swelling, fever, fall in blood pressure, skin rashes, wheals (raised red areas), flushing, headache). Sensations of hot and cold. Pain in the neck, back, bones and breast. Paleness. Light blue coloured lips and skin (because of too less oxygen in the blood).
If any of these side effects occurs, or if you notice any side effects not listed in this leaflet, please contact your doctor or a pharmacist.
5. How to store SmofKabiven
Keep out of the reach and sight of children.
Store in overpouch. Do not store above 25°C. Do not freeze.
Do not use after the expiry date which is written on the label on the bag and box. The expiry date refers to the last day of that month.
6. Further information
What SmofKabiven contains
The active substances are g per 1000 ml
Calcium chloride (as dihydrate) 0.28
Sodium glycerophosphate (as hydrate) 2.1
Magnesium sulphate (as heptahydrate) 0.61
Sodium acetate (as trihydrate) 1.7
Zinc sulphate (as heptahydrate) 0.0066
Medium-chain triglycerides 11.4
Fish oil, rich in omega-3-fatty acids 5.7
The other ingredients are: glycerol, purified egg phospholipids, all-rac-a-tocopherol, sodium hydroxide (pH-adjustment), sodium oleate, acetic acid (pH-adjustment), hydrochloric acid (pH-adjustment) and water for injections.
What SmofKabiven looks like and contents of the pack
Glucose- and amino acid solutions are clear, colourless or slightly yellow and free from particles. The lipid emulsion is white and homogenous.
Pack sizes:
1 x 986 ml, 4 x 986 ml 1 x 1477 ml, 4 x 1477 ml 1 x 1970 ml, 2 x 1970 ml 1 x 2463 ml, 2 x 2463 ml
Marketing Authorisation Holder and Manufacturer
To be completed nationally Manufacturer:
Fresenius Kabi AB, SE-751 74 Uppsala, Sweden
Marketing authorisation holder:
Fresenius Kabi
(country specific name and address will be added)
This leaflet was last approved in {MM/YYYY}. To be completed nationally
<-----------------------------------------------------------------------------------------------------------------------------
The following information is intended for medical or health care professionals only:
Warnings and precautions for use
To avoid risks associated with too rapid infusion rates, it is recommended to use a continuous and well-controlled infusion, if possible by using a volumetric pump.
Since an increased risk of infection is associated with the use of any central vein, strict aseptic precautions should be taken to avoid any contamination especially during catheter insertion.
Serum glucose, electrolytes and osmolarity as well as fluid balance, acid-base status and liver and enzyme tests should be monitored.
Any sign or symptom of anaphylactic reaction (such as fever, shivering, rash or dyspnoea) should lead to immediate interruption of the infusion.
SmofKabiven should not be given simultaneously with blood in the same infusion set due to the risk of pseudoagglutination.
Method of administration
Intravenous use, infusion into a central vein.
To provide total parenteral nutrition, trace elements, vitamins and possibly electrolytes (taking into account the electrolytes already present in SmofKabiven) should be added to SmofKabiven according to the patients need.
Infusion rate
The maximum infusion rate for glucose is 0.25 g/kg bw/h, for amino acid 0.1 g/kg bw/h, and for fat
0.15 g/kg bw/h.
The infusion rate should not exceed 2.0 ml/kg bw/h (corresponding to 0.25 g glucose, 0.10 g amino acids, and 0.08 g fat/kg bw/h). The recommended infusion period is 14-24 hours.
Precautions for disposal
Do not use if package is damaged.
Use only if the amino acid and glucose solutions are clear and colourless or slightly yellow and the lipid emulsion is white and homogenous. The contents of the three separate chambers have to be mixed before use, and before any additions are made via the additive port.
After separation of the peelable seals the bag should be inverted on a number of occasions to ensure a homogenous mixture, which does not show any evidence of phase separation.
For single use only. Any unused solution remaining after infusion should be discarded.
Compatibility
Only medicinal or nutrition solutions for which compatibility has been documented may be added to SmofKabiven. Compatibility for different additives and the storage time of the different admixtures will be available upon request.
Additions should be made aseptically.
Shelf-life after mixing
Chemical and physical in-use stability of the mixed three chamber bag has been demonstrated for 36 hours at 25 °C. From a microbiological point of view the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2-8°C.
Shelf-life after mixing with additives
From a microbiological point of view, the product should be used immediately when additions have been made. If not used immediately, the in-use storage time and conditions prior to use are the responsibility of the user. The storage time should normally not be longer than 24 hours at 2-8°C.
Instructions for use SmofKabiven
1. To remove the cover wrap hold the bag upright and tear from the notch along the upper edge, then simply tear open the long side, pull off the plastic covering and discard it along with the oxygen absorber.
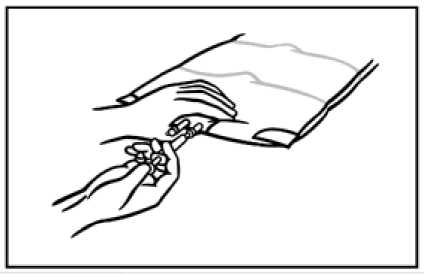
2. To mix the contents of the bag, place your fingertips on the upper compartment just on the seal as shown on the picture.
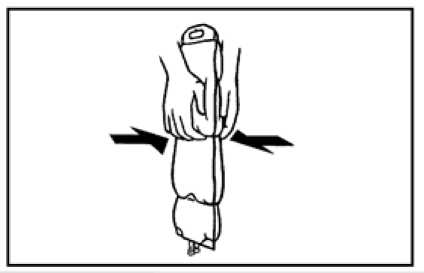
3. Grip the sides of the upper chamber with your fingertips and your thumbs and gently roll your knuckles together until the seal breaks.
Alternative technique (3): Put the bag, on a flat surface. Roll up the bag by using the handle until the seals are opened. Mix thoroughly by inverting the bag.
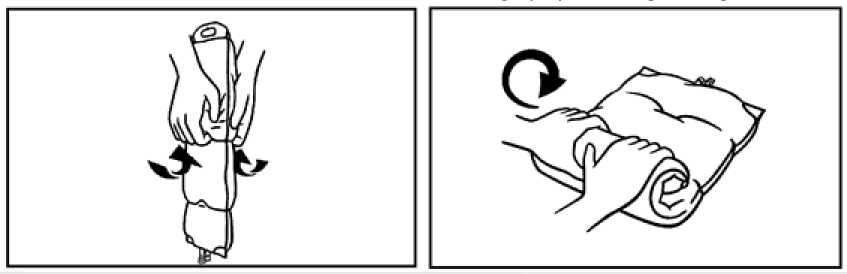
4. The remaining section of seal may now be gently teased apart.
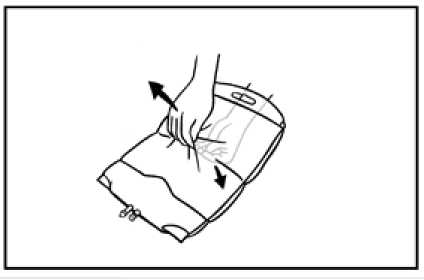
5. To peal open the lower seal, use the same technique as described above. Mix thoroughly by gently inverting the bag end-over-end several times.
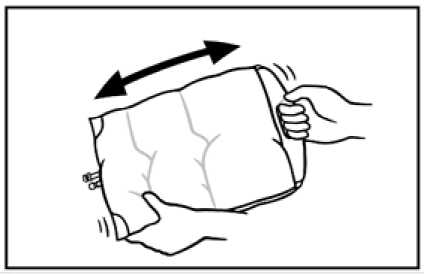
6. Before injecting additives swab the additive port with disinfectant.
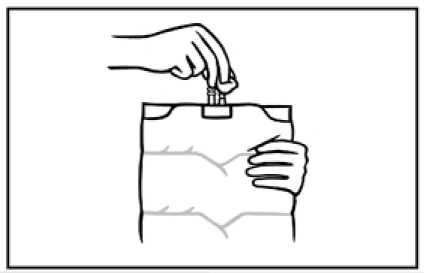
7. Support the base of the additive port. Fully insert the needle and inject the additives with known compatibility through the centre of the injection site. Mix thoroughly between each addition by inverting the
bag several times.
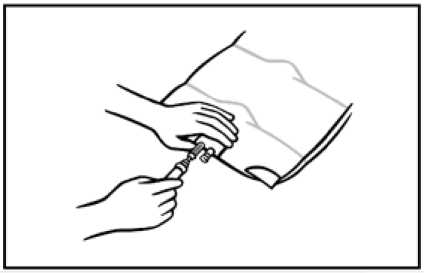
8. Use a non-vented infusion set or close the air-inlet on a vented set. Remove the set port cover by pulling the ring upwards. Support the base of the infusion port. Insert the spike straight into the infusion port. Twist and push the spike through the diagram. The spike should be fully inserted to secure it in place.
7