Sodium Chloride 0.9% Solution For Injection

FRESENIUS
KABI
Anexo 6.00 do PT.G.087
|
CODIGO: Code |
798245 |
DESIGNA^AO: Name |
LIT.CL SODIO 0,9% INJ PE FKUK |
ELABORADO POR: Made by |
JD0686 |
|
VERSAO: Version |
/XX |
PROVA: Proof |
05 |
DATA: Date |
15 Jul.2016 |
|
FICHA TECNICA: Technical Sheet |
798245 |
ESCALA: Scale |
1:1 |
Text size: 8 pts |
|
CORES: |
• Black |
|
Colours |
• Cut |
|
o | |
|
o | |
|
Cl |
Package leaflet: Information for the user
Sodium Chloride 0.9% Solution for injection
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have further questions, please ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
The name of your medicine is "Sodium Chloride 0.9% Solution for injection" but it will be referred to as Sodium Chloride 0.9% throughout this leaflet.
What is in this leaflet
1. What Sodium Chloride 0.9% is and what it is used for
2. What you need to know before you use Sodium Chloride 0.9%
3. How to use Sodium Chloride 0.9%
4. Possible side effects
5. How to store Sodium Chloride 0.9%
6. Contents of the pack and other information
1. WHAT SODIUM CHLORIDE 0.9% IS AND WHAT IT IS USED FOR
Sodium Chloride 0.9% is a solution for injection and is used as a solvent and carrier for drugs. It can be given by the intravenous, intramuscular or subcutaneous route.
This product belongs to the "Electrolyte Solutions" group and is distributed under medical prescription.
B 300832/01 V003/LB
2. WHAT YOU NEED TO KNOW BEFORE YOU USE SODIUM CHLORIDE 0.9%
Do not use Sodium Chloride 0.9%:
• if you are allergic to sodium chloride or any of the other ingredients of this medicine (listed in section 6).
Other medicines and Sodium Chloride 0.9%
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, even those not prescribed. The solution of Sodium Chloride 0.9% shows no incompatibilities with other medicinal products.
Sodium Chloride 0.9% with food and drink
Sodium Chloride 0.9% is not known to have any interactions
when given at the same time as food and drink.
Pregnancy and breast-feeding
Please ask your doctor or your pharmacist before being administered this medicine.
If a correct and controlled dose of Sodium Chloride 0.9% is administered, no effects on pregnant women and during lactation are expected.
Driving and using machines
There is no evidence that Sodium Chloride 0.9% solution may affect the ability to drive or to use machines.
3. HOW TO USE SODIUM CHLORIDE 0.9%
Sodium Chloride 0.9% will be given to you by an appropriately trained professional.
Sodium Chloride 0.9% will be administered by intravenous, intramuscular or subcutaneous route.
It does not contain any type of preservative or germicide, hence, opened ampoules should be immediately discarded once used. The amount used will vary depending on the concentration at which the drug to be dissolved is to be administered.
Your doctor will inform you of the duration of your treatment.
SUMMARYOF PRODUCT CHARACTERISTICS
1. NAME OF THE MEDICINAL PRODUCT
Sodium Chloride 0.9% Solution for injection
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Sodium chloride 0.9%w/v
Electrolytes: Na+ 154 mmol/l Cl- 154 mmol/l For a full list of excipients, see section 6.1
3. PHARMACEUTICAL FORM
Solution for Injection A clear colourless solution
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
Sodium Chloride 0.9% Solution for injection is used as a solvent and carrier solution for injectable drugs.
4.2 Posology and method of administration
Posoloav As required
Method of administration
Intravenous, intramuscular or subcutaneous use
4.3 Contraindications
None stated.
4.4 Special warnings and precautions for use
If Sodium Chloride 0.9% Solution for injection is to be administered subcutaneously be aware that any additions to the isotonic normal saline solution could render it hypertonic and thus cause pain at the injection site.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed.
4.6 Fertility, pregnancy and lactation None stated
4.7 Effects on ability to drive and use machines
No studies on the ability to drive and use machines have been performed.
4.8 Undesirable effects
None stated
Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
No particulars stated.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties ATC code: B05XA03
Sodium chloride 0.9% solution shows the same osmotic pressure as body fluids.
The isotonic solution of sodium chloride is a suitable vehicle for administration of a large number of drugs and electrolytes.
5.2 Pharmacokinetic properties
Sodium and chloride electrolytes distribute, primarily, in the extracellular fluid. Because the physiological saline solution is isotonic, its administration will not produce a change in the osmotic pressure of the extracellular fluid, whereby water will not pass into the intracellular compartment and both ions will practically enter the cell.
5.3 Preclinical safety data
Safety of sodium chloride isotonic solutions is adequately recognised in the fluid therapy field worldwide, thanks to a wide experience available in the use of this solution as a restorer of the hydro-electrolytic balance.

If you have received more Sodium Chloride 0.9% than you should:
Given the product's nature, if its indication and administration are correct and controlled, there is no risk of overdose.
If you should have been given more Sodium Chloride 0.9% than you should have had, inform your doctor immediately.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everyone gets them.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via Yellow Card System at: www. mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE SODIUM CHLORIDE 0.9%
Keep this medicine out of the sight and reach of children.
For single use only.
To be used immediately after the ampoule is opened.
Do not use after expiry date which is stated on the carton after EXP. The expiry date refers to the last day of that month.
Any unused solution should be discarded.
Use only clear, particle-free solutions and undamaged containers. Do not throw away any medicines via wastewater. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Sodium Chloride 0.9% contains
Each 1 ml contains 9 mg of sodium chloride.
The other ingredients are water for injection and traces of sodium hydroxide and hydrochloric acid.
What Sodium Chloride 0.9% looks like and contents of the pack.
Sodium Chloride 0.9% is a clear colourless, particle-free solution. It is available in the following formats:
Carton with 20 ampoules of 5 ml.
Carton with 20 ampoules of 10 ml.
Carton with 20 ampoules of 20 ml.
Marketing Authorisation Holder
Fresenius Kabi Limited
Cestrian Court
Eastgate Way
Manor Park
Runcorn
Cheshire
WA7 1NT.
UK.
Manufacturer:
LABESFAL - Laboratories Almiro S.A. FRESENIUS KABI GROUP Zona Industrial do Lagedo 3465-157 Santiago de Besteiros, PORTUGAL
This leaflet was last revised in 07/2016
FRESENIUS
KABI
798245/XX
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Water for Injection.
Sodium hydroxide.
Hydrochloric acid.
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
a) Shelf life of the product as packaged for sale:
Shelf life of the pharmaceutical product: 2 years
b) Shelf life after first opening of the container:
The product should be used immediately after opening.
6.4 Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5 Nature and contents of container
Low density polyethylene ampoules (LDPE).
- Carton with 20 ampoules of 5 ml
- Carton with 20 ampoules of 10 ml
- Carton with 20 ampoules of 20 ml
6.6 Special precautions for disposal and other handling
It is not necessary to sterilise the bottle before its opening.
It is not necessary to use any cutting element to open the ampoule.
Once the ampoule is opened the top of it can perfectly be adjusted to the syringe cone (cone Luer), with which it is necessary to use the needle.
Handling instructions:
To break off a single ampoule, twist one ampoule against the remaining ampoules of the pack without touching the head and neck of the ampoules (1). Shake the ampoule with one single movement as shown below in order to remove the liquid kept in the cap (2). To open the ampoule, twist the ampoule body and the ampoule head in opposite directions until the neck breaks off (3). Connect the ampoule to the luer-syringe or luer-lock syringe as shown in figure (4).
|
1 |
2 |
3 |
4 |
|
-nnnqjF | |||
|
/ ^ \ | |||
|
^ \ l |
\ |
n |
Therefore, no needle is needed to extract the solution. Extract the liquid.
The solution does not contain any type of preservative or bactericide, so open and unused ampoules should be discarded immediately.
7. MARKETING AUTHORISATION HOLDER
Fresenius Kabi Limited
Cestrian Court
Eastgate Way
Manor Park
Runcorn
Cheshire
WA71NT
UK
8. MARKETING AUTHORISATION NUMBER
PL 08828/0178
9. DATE OF FIRST AUTHORISATION/ RENEWAL OF AUTHORISATION
Date of first authorisation: 30/08/2007 Date of last renewal:
10. DATE OF REVISION OF THE TEXT
07/2016 798245/XX
Package leaflet: Information for the user
Sodium Chloride 0.9% Solution for injection
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have further questions, please ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
The name of your medicine is "Sodium Chloride 0.9% Solution for injection" but it will be referred to as Sodium Chloride 0.9% throughout this leaflet.
What is in this leaflet
1. What Sodium Chloride 0.9% is and what it is used for
2. What you need to know before you use Sodium Chloride 0.9%
3. How to use Sodium Chloride 0.9%
4. Possible side effects
5. How to store Sodium Chloride 0.9%
6. Contents of the pack and other information
1. WHAT SODIUM CHLORIDE 0.9% IS AND WHAT IT IS USED FOR
Sodium Chloride 0.9% is a solution for injection and is used as a solvent and carrier for drugs. It can be given by the intravenous, intramuscular or subcutaneous route.
This product belongs to the "Electrolyte Solutions" group and is distributed under medical prescription.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE SODIUM CHLORIDE 0.9%
S-c_ — — — — — — — — —
Do not use Sodium Chloride 0.9%:
• If you are allergic to sodium chloride or any of the other ingredients of this medicine (listed in section 6).
Other medicines and Sodium Chloride 0.9%
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, even those not prescribed.
The solution of Sodium Chloride 0.9% shows no incompatibilities with other medicinal products.
Sodium Chloride 0.9% with food and drink
Sodium Chloride 0.9% is not known to have any interactions when given at the same time as food and drink.
Pregnancy and breast-feeding
Please ask your doctor or your pharmacist before being administered this medicine.
If a correct and controlled dose of Sodium Chloride 0.9% is administered, no effects on pregnant women and during lactation are expected.
Driving and using machines
There is no evidence that Sodium Chloride 0.9% solution may affect the ability to drive or to use machines.
3. HOW TO USE SODIUM CHLORIDE 0.9%
Sodium Chloride 0.9% will be given to you by an appropriately trained professional.
Sodium Chloride 0.9% will be administered by intravenous, intramuscular or subcutaneous route.
It does not contain any type of preservative or germicide, hence, opened ampoules should be immediately discarded once used.
The amount used will vary depending on the concentration at which the drug to be dissolved is to be administered.
Your doctor will inform you of the duration of your treatment.
If you have received more Sodium Chloride 0.9% than you should:
Given the product's nature, if its indication and administration are correct and controlled, there is no risk of overdose.
If you have been given more Sodium Chloride 0.9% than you should have had, inform your doctor immediately.
V003/VS (BP8)
SUMMARY OF PRODUCT CHARACTERISTICS
1. NAME OF THE MEDICINAL PRODUCT
Sodium Chloride 0.9% Solution for injection
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Sodium chloride 0.9%w/v Electrolytes:
Na+ 154 mmol/l Cl- 154 mmol/l
For a full list of excipients, see section 6.1
3. PHARMACEUTICAL FORM
Solution for Injection A clear colourless solution
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
Sodium Chloride 0.9% Solution for injection is used as a solvent and carrier solution for injectable drugs.
4.2 Posology and method of administration
Posology As required
Method of administration
Intravenous, intramuscular or subcutaneous use
4.3 Contraindications
None stated.
4.4 Special warnings and precautions for use
If Sodium Chloride 0.9% Solution for injection is to be administered subcutaneously be aware that any additions to the isotonic normal saline solution could render it hypertonic and thus cause pain at the injection site.
B306142/02 V003/VS (BP8)
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed.
4.6 Fertility, pregnancy and lactation
None stated
4.7 Effects on ability to drive and use machines
No studies on the ability to drive and use machines have been performed.
4.8 Undesirable effects
None stated.
Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
No particulars stated.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties ATC code: B05XA03
Sodium chloride 0.9% solution shows the same osmotic pressure as body fluids.
The isotonic solution of sodium chloride is a suitable vehicle for administration of a large number of drugs and electrolytes.
5.2 Pharmacokinetic properties
Sodium and chloride electrolytes distribute, primarily, in the extracellular fluid. Because the physiological saline solution is isotonic, its administration will not produce a change in the osmotic pressure of the extracellular fluid, whereby water will not pass into the intracellular compartment and both ions will practically enter the cell.
5.3 Preclinical safety data
Safety of sodium chloride isotonic solutions is adequately recognised in the fluid therapy field worldwide, thanks to a wide experience available in the use of this solution as a restorer of the hydro-electrolytic balance.
Like all medicines, this medicine can cause side effects, although not everyone gets them.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via Yellow Card System at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE SODIUM CHLORIDE 0.9%
Keep this medicine out of the sight and reach of children.
For single use only.
To be used immediately after the ampoule is opened.
Do not use after expiry date which is stated on the carton after EXP. The expiry date refers to the last day of that month. Any unused solution should be discarded.
Use only clear, particle-free solutions and undamaged containers.
Do not throw away any medicines via wastewater. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
Carton with 20 ampoules of 5 ml. Carton with 20 ampoules of 10 ml. Carton with 20 ampoules of 20 ml.
Marketing Authorisation Holder
Fresenius Kabi Limited Cestrian Court Eastgate Way Manor Park Runcorn Cheshire WA7 1NT.
UK.
Manufacturer
FRESENIUS KABI ESPANA, S.A C/ Marina, 16-18, planta 17 08005-Barcelona (ESPANA)
This leaflet was last revised in 07/2016
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Sodium Chloride 0.9% contains
Each 1 ml contains 9 mg of sodium chloride.
The other ingredients are water for injection and traces of sodium hydroxide and hydrochloric acid.
What Sodium Chloride 0.9% looks like and contents of the pack.
Sodium Chloride 0.9% is a clear colourless, particle-free solution. It is available in the following formats:
— — — — — — — — — —
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Water for Injection.
Sodium hydroxide.
Hydrochloric acid.
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
a) Shelf life of the product as packaged for sale:
Shelf life of the pharmaceutical product: 2 years
b) Shelf life after first opening of the container:
The product should be used immediately after opening.
6.4 Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5 Nature and contents of container
Low density polyethylene ampoules (LDPE).
- Carton with 20 ampoules of 5 ml
- Carton with 20 ampoules of 10 ml
- Carton with 20 ampoules of 20 ml
6.6 Special precautions for disposal and other handling
It is not necessary to sterilise the bottle before its opening.
It is not necessary to use any cutting element to open the ampoule. Once the ampoule is opened the top of it can perfectly be adjusted to the syringe cone (cone Luer), with which it is necessary to use the needle.
Handling instructions:
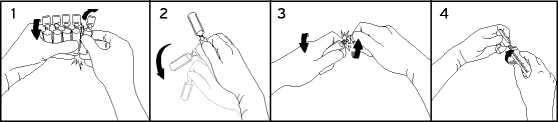
To break off a single ampoule, twist one ampoule against the remaining ampoules of the pack without touching the head and neck of the ampoules (1). Shake the ampoule with one single movement as shown below in order to remove the liquid kept in the cap (2). To open the ampoule, twist the ampoule body and ampoule head in opposite directions until the neck breaks off (3). Connect the ampoule to the luer-syringe or luer-lock syringe as shown in figure (4).
FRESENIUS
KABI
The solution does not contain any type of preservative or bactericide, so open and unused ampoules should be discarded immediately.
7. MARKETING AUTHORISATION HOLDER
Fresenius Kabi Limited Cestrian Court Eastgate Way Manor Park Runcorn Cheshire WA71NT UK
8. MARKETING AUTHORISATION NUMBER
PL 08828/0178
9. DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
Date of first authorisation: 30/08/2007 Date of last renewal:
10. DATE OF REVISION OF THE TEXT
07/2016
Sodium Chloride (BP8) B306142/02
Colour: Black Size: 297 x 210 mm Fonts size: 11/8 pt 20.07.2016 Correction: 1 Version: 02
Paper weight: 60 gr/m2
Package leaflet: Information for the user
Sodium Chloride 0.9% Solution for injection
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have further questions, please ask your doctor, pharmacist or nurse.
• If you get any side effects, talk to your doctor, or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
The name of your medicine is "Sodium Chloride 0.9% Solution for injection" but it will be referred to as Sodium Chloride 0.9% throughout this leaflet.
What is in this leaflet
1. What Sodium Chloride 0.9% is and what it is used for
2. What you need to know before you use Sodium Chloride 0.9%
3. How to use Sodium Chloride 0.9%
4. Possible side effects
5. How to store Sodium Chloride 0.9%
6. Contents of the pack and other information
1. WHAT SODIUM CHLORIDE 0.9% IS AND WHAT IT IS USED FOR
Sodium Chloride 0.9% is a solution for injection and is used as a solvent and carrier for drugs. It can be given by the intravenous, intramuscular or subcutaneous route.
This product belongs to the "Electrolyte Solutions" group and is distributed under medical prescription.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE SODIUM CHLORIDE 0.9%
S-c_ — — — — — — — — —
Do not use Sodium Chloride 0.9%:
• If you are allergic to sodium chloride or any of the other ingredients of this medicine (listed in section 6).
Other medicines and Sodium Chloride 0.9%
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, even those not prescribed.
The solution of Sodium Chloride 0.9% shows no incompatibilities with other medicinal products.
Sodium Chloride 0.9% with food and drink
Sodium Chloride 0.9% is not known to have any interactions when given at the same time as food and drink.
Pregnancy and breast-feeding
Please ask your doctor or your pharmacist before being administered this medicine.
If a correct and controlled dose of Sodium Chloride 0.9% is administered, no effects on pregnant women and during lactation are expected.
Driving and using machines
There is no evidence that Sodium Chloride 0.9% solution may affect the ability to drive or to use machines.
3. HOW TO USE SODIUM CHLORIDE 0.9%
Sodium Chloride 0.9% will be given to you by an appropriately trained professional.
Sodium Chloride 0.9% will be administered by intravenous, intramuscular or subcutaneous route.
It does not contain any type of preservative or germicide, hence, opened ampoules should be immediately discarded once used.
The amount used will vary depending on the concentration at which the drug to be dissolved is to be administered.
Your doctor will inform you of the duration of your treatment.
If you have received more Sodium Chloride 0.9% than you should:
Given the product's nature, if its indication and administration are correct and controlled, there is no risk of overdose.
If you have been given more Sodium Chloride 0.9% than you should have had, inform your doctor immediately.
V003/VS (BP9)
SUMMARY OF PRODUCT CHARACTERISTICS
1. NAME OF THE MEDICINAL PRODUCT
Sodium Chloride 0.9% Solution for injection
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Sodium chloride 0.9%w/v Electrolytes:
Na+ 154 mmol/l Cl- 154 mmol/l
For a full list of excipients, see section 6.1
3. PHARMACEUTICAL FORM
Solution for Injection A clear colourless solution
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
Sodium Chloride 0.9% Solution for injection is used as a solvent and carrier solution for injectable drugs.
4.2 Posology and method of administration
Posology As required
Method of administration
Intravenous, intramuscular or subcutaneous use
4.3 Contraindications
None stated.
4.4 Special warnings and precautions for use
If Sodium Chloride 0.9% Solution for injection is to be administered subcutaneously be aware that any additions to the isotonic normal saline solution could render it hypertonic and thus cause pain at the injection site.
B306267/02 V003/VS (BP9)
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed.
4.6 Fertility, pregnancy and lactation
None stated
4.7 Effects on ability to drive and use machines
No studies on the ability to drive and use machines have been performed.
4.8 Undesirable effects
None stated.
Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
No particulars stated.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties ATC code: B05XA03
Sodium chloride 0.9% solution shows the same osmotic pressure as body fluids.
The isotonic solution of sodium chloride is a suitable vehicle for administration of a large number of drugs and electrolytes.
5.2 Pharmacokinetic properties
Sodium and chloride electrolytes distribute, primarily, in the extracellular fluid. Because the physiological saline solution is isotonic, its administration will not produce a change in the osmotic pressure of the extracellular fluid, whereby water will not pass into the intracellular compartment and both ions will practically enter the cell.
5.3 Preclinical safety data
Safety of sodium chloride isotonic solutions is adequately recognised in the fluid therapy field worldwide, thanks to a wide experience available in the use of this solution as a restorer of the hydro-electrolytic balance.
Like all medicines, this medicine can cause side effects, although not everyone gets them.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via Yellow Card System at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE SODIUM CHLORIDE 0.9%
Keep this medicine out of the sight and reach of children.
For single use only.
To be used immediately after the ampoule is opened.
Do not use after expiry date which is stated on the carton after EXP. The expiry date refers to the last day of that month. Any unused solution should be discarded.
Use only clear, particle-free solutions and undamaged containers.
Do not throw away any medicines via wastewater. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
Carton with 20 ampoules of 5 ml. Carton with 20 ampoules of 10 ml. Carton with 20 ampoules of 20 ml.
Marketing Authorisation Holder
Fresenius Kabi Limited Cestrian Court Eastgate Way Manor Park Runcorn Cheshire WA7 1NT.
UK.
Manufacturer
FRESENIUS KABI ESPANA, S.A C/ Marina, 16-18, planta 17 08005-Barcelona (ESPANA)
This leaflet was last revised in 07/2016
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Sodium Chloride 0.9% contains
Each 1 ml contains 9 mg of sodium chloride.
The other ingredients are water for injection and traces of sodium hydroxide and hydrochloric acid.
What Sodium Chloride 0.9% looks like and contents of the pack.
Sodium Chloride 0.9% is a clear colourless, particle-free solution. It is available in the following formats:
— — — — — — — — — —
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Water for Injection.
Sodium hydroxide.
Hydrochloric acid.
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
a) Shelf life of the product as packaged for sale:
Shelf life of the pharmaceutical product: 2 years
b) Shelf life after first opening of the container:
The product should be used immediately after opening.
6.4 Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5 Nature and contents of container
Low density polyethylene ampoules (LDPE).
- Carton with 20 ampoules of 5 ml
- Carton with 20 ampoules of 10 ml
- Carton with 20 ampoules of 20 ml
6.6 Special precautions for disposal and other handling
It is not necessary to sterilise the bottle before its opening.
It is not necessary to use any cutting element to open the ampoule. Once the ampoule is opened the top of it can perfectly be adjusted to the syringe cone (cone Luer), with which it is necessary to use the needle.
Handling instructions:
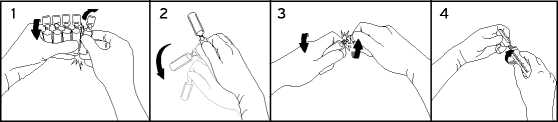
To break off a single ampoule, twist one ampoule against the remaining ampoules of the pack without touching the head and neck of the ampoules (1). Shake the ampoule with one single movement as shown below in order to remove the liquid kept in the cap (2). To open the ampoule, twist the ampoule body and ampoule head in opposite directions until the neck breaks off (3). Connect the ampoule to the luer-syringe or luer-lock syringe as shown in figure (4).
FRESENIUS
KABI
The solution does not contain any type of preservative or bactericide, so open and unused ampoules should be discarded immediately.
7. MARKETING AUTHORISATION HOLDER
Fresenius Kabi Limited Cestrian Court Eastgate Way Manor Park Runcorn Cheshire WA71NT UK
8. MARKETING AUTHORISATION NUMBER
PL 08828/0178
9. DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
Date of first authorisation: 30/08/2007 Date of last renewal:
10. DATE OF REVISION OF THE TEXT
07/2016
Sodium Chloride (BP9) B306267/02
Colour: Black
Size: 297 x 210 mm
Size folding: 50 x 210 mm
Fonts size: 11/8 pt
20.07.2016
Correction: 1
Version: 02
Paper weight: 60 gr/m2