Sodium Chloride 0.9% W/V Nebuliser Solution
Ref: LTT155/280714/1 /F
Saline® Steri-Neb 0.9% w/v Nebuliser Solution (sodium chloride)
Patient Information Leaflet
A /
Read all of this leaflet carefully before you start
using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Your medicine is called Saline Steri-Neb 0.9% w/v
Nebuliser solution but will be referred to as Saline
Steri-Neb throughout the leaflet.
In this leaflet:
What Saline Steri-Neb is and what it is used for
[a Before you use Saline Steri-Neb How to use Saline Steri-Neb Possible side effects .5 How to store Saline Steri-Neb ^ Further information
^ What Saline Steri-Neb is and what it is used for
Saline Steri-Neb is used to dilute nebuliser solutions. The nebuliser turns the solution into a fine mist which you can breathe in.
You should carefully read the leaflet supplied with the nebuliser solutions before using them with Saline Steri-Neb.
[2) Before you use Saline Steri-Neb
Do NOT use Saline Steri-Neb if you:
• are allergic (hypersensitive) to sodium chloride or any of the other ingredients of this medicine.
Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy and breast-feeding
If you are pregnant, planning to become pregnant or breast-feeding, ask your doctor for advice before taking any medicine.
Driving and using machines
Saline Steri-Neb is not expected to affect your ability to drive or operate machinery.
How to use Saline Steri-Neb
Always use Saline Steri-Neb exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
You must only use Saline Steri-Neb in a nebuliser. Do not swallow the solution or use it in injections.
Read the manufacturer's instructions and make sure you know how to use the nebuliser before you take your medicine.
• Prepare your nebuliser for use according to the manufacturer's instructions
• Empty the solution that needs diluting into the nebuliser following the manufacturer's instructions
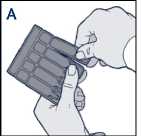
• Remove a Steri-Neb from the labelled strip by twisting and pulling (diagram A)
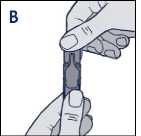
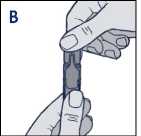
• Hold the Steri-Neb upright and twist off the cap (diagram B)
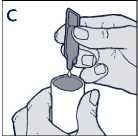
• Squeeze the contents into the reservoir of your nebuliser (diagram C)
• Following the manufacturer's instructions, use your nebuliser
• After you have used your nebuliser, throw away any solution that is left in the reservoir
• Clean your nebuliser thoroughly.
Adults, children and the elderly
Follow your doctor's instructions when you are diluting nebuliser solutions.
Mix up a fresh batch of the solution before each dose and use as directed by your doctor.
If you use more Saline Steri-Neb than you should
If you (or someone else) swallow any of the solution, or if you think a child has swallowed any of the solution, contact your nearest hospital casualty department or your doctor immediately. Please take this leaflet, any remaining solution, and the container with you to the hospital or doctor so that they know which solution has been consumed.
If you forget to use Saline Steri-Neb
If you forget to use the nebuliser, use it as soon as you remember, unless it is nearly time to use it again. Do not take a double dose of your medicine to make up for a forgotten dose.
If you stop using Saline Steri-Neb
Do not stop taking your medicine without talking to your doctor first even if you feel better.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Q Possible side effects
Like all medicines, the medicine you breathe in with Saline Steri-Neb could cause some side effects, although not everybody gets them. The leaflet that comes with that medicine will give you more information on this.
Tell your doctor if you notice any side effects. Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
• Do not store above 25°C.
• Do not refrigerate or freeze
• KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
• Use immediately after opening the ampoule. Discard any unused portion.
• Do not use this medicine after the expiry date which is stated on the carton and Steri-Neb unit. The expiry date refers to the last day of the month.
• If your medicine becomes discoloured or shows any other signs of deterioration, consult your pharmacist who will tell you what to do.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Q Further Information
What Saline Steri-Neb contains
Each Saline Steri-Neb contains 2.5ml of a 0.9% w/v solution of sodium chloride. The other ingredient is water for injections.
What Saline Steri-Neb looks like and contents of the pack
Saline Steri-Neb is Clear, colourless solution in a clear, plastic single dose ampoule.
Available in packs of 20 ampoules.
Manufacturer and Licence Holder
This medicine is manufactured by Ivax Pharmaceuticals UK, Aston Lane North, Whitehouse Vale industrial Estate, Preston Brook, Runcorn, Cheshire, WA7 3FA, UK and is procured from within the EU. Product Licence Holder: LTT Pharma Limited, Unit 18, Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 0RE and repackaged by Lexon (UK) Limited, B98 0RE.
POM PL 33723/0155
Leaflet revision date: 28/07/14
Saline is a registered trademark of Norton Healthcare Limited.
Blind or partially sighted?
Is this leaflet hard to see or read? Phone Lexon (UK) Limited,
Tel: 01527 505414 for help.
Ref: LTT155/280714/2/F
Sodium chloride 0.9% w/v Nebuliser Solution
Patient Information Leaflet
Read all of this leaflet carefully before you start
using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Your medicine is called Sodium Chloride 0.9% w/v
Nebuliser solution but will be referred to as Sodium
Chloride throughout the leaflet.
In this leaflet:
^ What Sodium Chloride is and what it is used for
[a Before you use Sodium Chloride How to use Sodium Chloride Possible side effects [5 How to store Sodium Chloride l6 Further information
^ What Sodium Chloride is and what it is used for
Sodium Chloride is used to dilute nebuliser solutions. The nebuliser turns the solution into a fine mist which you can breathe in.
You should carefully read the leaflet supplied with the nebuliser solutions before using them with Sodium Chloride.
w Before you use Sodium Chloride
Do NOT use Sodium Chloride if you:
• are allergic (hypersensitive) to sodium chloride or any of the other ingredients of this medicine.
Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy and breast-feeding
If you are pregnant, planning to become pregnant or breast-feeding, ask your doctor for advice before taking any medicine.
Driving and using machines
Sodium Chloride is not expected to affect your ability to drive or operate machinery.
How to use Sodium Chloride
Always use Sodium Chloride exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
You must only use Sodium Chloride in a nebuliser. Do not swallow the solution or use it in injections.
Read the manufacturer's instructions and make sure you know how to use the nebuliser before you take your medicine.
• Prepare your nebuliser for use according to the manufacturer's instructions
• Empty the solution that needs diluting into the nebuliser following the manufacturer's instructions
• Remove a ampoule from the labelled strip by twisting and pulling (diagram A)
• Hold the ampoule upright and twist off the cap (diagram B)
• Squeeze the contents into the reservoir of your nebuliser (diagram C)
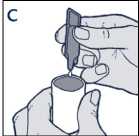
• Following the manufacturer's instructions, use your nebuliser
• After you have used your nebuliser, throw away any solution that is left in the reservoir
• Clean your nebuliser thoroughly.
Adults, children and the elderly
Follow your doctor's instructions when you are diluting nebuliser solutions.
Mix up a fresh batch of the solution before each dose and use as directed by your doctor.
If you use more Sodium Chloride than you should
If you (or someone else) swallow any of the solution, or if you think a child has swallowed any of the solution, contact your nearest hospital casualty department or your doctor immediately. Please take this leaflet, any remaining solution, and the container with you to the hospital or doctor so that they know which solution has been consumed.
If you forget to use Sodium Chloride
If you forget to use the nebuliser, use it as soon as you remember, unless it is nearly time to use it again. Do not take a double dose of your medicine to make up for a forgotten dose.
If you stop using Sodium Chloride
Do not stop taking your medicine without talking to your doctor first even if you feel better.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Q Possible side effects
Like all medicines, the medicine you breathe in with Sodium Chloride could cause some side effects, although not everybody gets them. The leaflet that comes with that medicine will give you more information on this.
Tell your doctor if you notice any side effects. Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
• Do not store above 25°C.
• Do not refrigerate or freeze
• KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
• Use immediately after opening the ampoule. Discard any unused portion.
• Do not use this medicine after the expiry date which is stated on the carton and ampoule unit. The expiry date refers to the last day of the month.
• If your medicine becomes discoloured or shows any other signs of deterioration, consult your pharmacist who will tell you what to do.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Q Further Information
What Sodium Chloride contains
Each Sodium Chloride contains 2.5ml of a 0.9% w/v solution of sodium chloride. The other ingredient is water for injections.
What Sodium Chloride looks like and contents of the pack
Sodium Chloride is Clear, colourless solution in a clear, plastic single dose ampoule.
Available in packs of 20 ampoules.
Manufacturer and Licence Holder
This medicine is manufactured by Ivax Pharmaceuticals UK, Aston Lane North, Whitehouse Vale industrial Estate, Preston Brook, Runcorn, Cheshire, WA7 3FA, UK and is procured from within the EU. Product Licence Holder: LTT Pharma Limited, Unit 18, Oxleasow Road, East Moons Moat, Redditch, Worcestershire, B98 0RE and repackaged by Lexon (UK) Limited, B98 0RE.
POM PL 33723/0155
Leaflet revision date: 28/07/14
Blind or partially sighted?
Is this leaflet hard to see or read? Phone Lexon (UK) Limited,
Tel: 01527 505414 for help.
Ref: LTT155/280714/1 /B