Sodium Feredetate 190Mg/5Ml Oral Solution
= cutter line
= printable area
- =perfline
190mg/5ml ) Oral Solution
Each 5ml dose contains Sodium Feredetate 190mg (equivalentto 27.5mgofelemental iron/5ml)
Also contains sorbitol (E420), methyl hydroxybenzoate (E218), propyl hydroxybenzoate (E216), ponceau 4R lake (E124) and ethanol.
For oral administration. Read the package leaflet before use. Keep out of the sight and reach of children.
Important warning: Contains iron. Keep out of the sight and reach of children, as overdose may be fatal.
Store in the original package in order to protect from light. Use with in three months of opening.
Use as directed by your physician.
500ml Solution NRlM
[poM PL 41830/0037 PLHolder:
NRIM Limited Unit 15Moorcroft Harlington Road Hillingdon UB83HD UK
~s~
2
f
I90mg/5ml ) Oral Solution
Each 5ml dose contains Sodium Feredetate 190mg I(equivalentto27.5mgofelementaliron/5ml) MJ
Also contains sorbitol (E420), methyl hydroxybenzoate (E218), ipropyl hydroxybenzoate (E216), ponceau 4R lake (E124) and ethanol.
For oral administration. Read the package leaflet before use. Keep out of the sight and reach of children.
Important warning: Contains iron. Keep out of the sight and reach of children, as overdose may be fatal.
Store in the original package in order to protect from light. Use with in three months of opening.
[POM PL 41830/0037 PL Holder:
NRIM Limited Unit 15Moorcroft Harlington Road Hillingdon UB8 3HD UK
Use as directed by your physician.
500ml Solution _ _NRlM
|
/ |
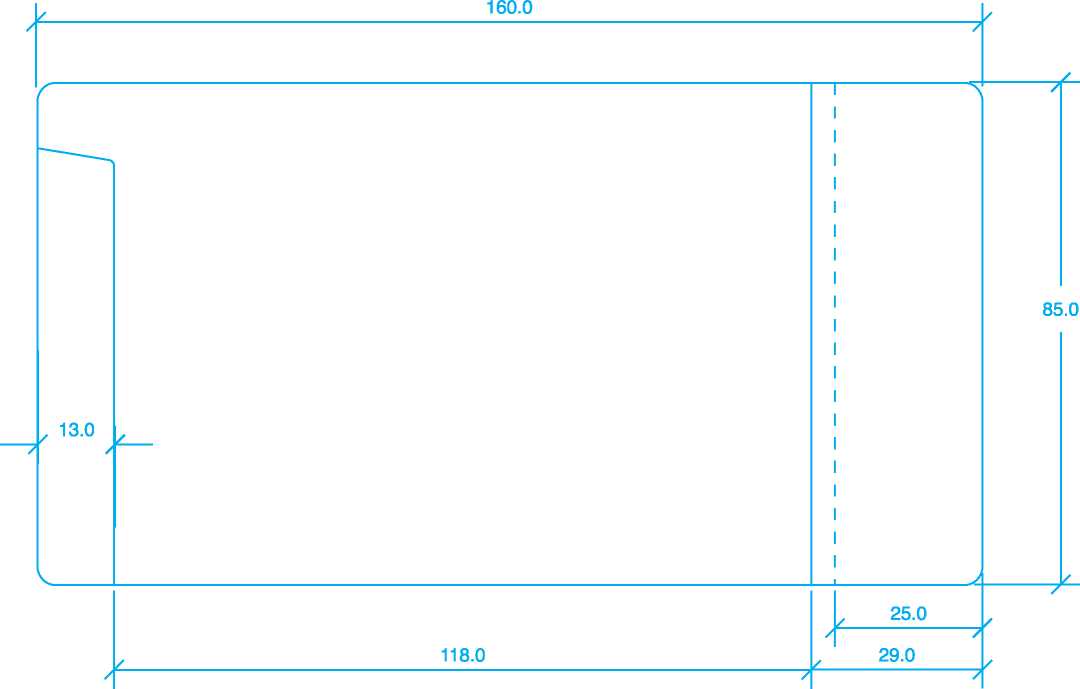
118.0 | |
|
7 | ||
PACKAGE LEAFLET: INFORMATION FOR THE USER
SODIUM FEREDETATE 190 MG/5 ML ORAL SOLUTION
i
(equivalent to 27.5 mg of elemental iron/5ml)
i
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even i their signs of illness are the same as yours.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
I
What
What Sodium Feredetate is and what it is used for
What you need to know before you take Sodium Feredetate
How to take Sodium Feredetate
Possible side effects
How to store Sodium Feredetate
Contents of the pack and other information
1. WHAT SODIUM FEREDETATE IS AND WHAT IT IS USED FOR
The name of your medicine is Sodium Feredetate 190 mg/5 ml Oral Solution (called Sodium Feredetate throughout this leaflet).
Sodium Feredetate is used to treat anaemia caused by too little iron in the body (iron deficiency anaemia). The form of iron used in this product means that it is less likely to cause stomach upsets than other iron-containing medicines, and will not discolour teeth.
It is to be taken by:
pregnant women when other forms of oral iron may not be well tolerated
-I
2. WHAT YOU NEED TO KNOW BEFORE YOU TAKE SODIUM FEREDETATE
children and adults who have become anaemic as a result of having rheumatoid arthritis.
Do NOT take Sodium Feredetate and tell your doctor if you:
are allergic to sodium feredetate (also known as sodium iron edetate) or any of the ingredients of this medicine (listed in section 2 and 6) have a history of sensitivity to iron-containing preparations.
have a disorder in which there is excessive absorption and storage of (iron haemochromatosis or haemosiderosis).
have repeated blood transfusions or have had them in the past.
• are currently having iron injections.
Talk to your doctor or pharmacist before using Sodium Feredetate:
I- if you have haemolytic anaemia - if you have an iron storage or absorption disease
-—if you Jaave-gastroi^testinaLdisease. ________________
|
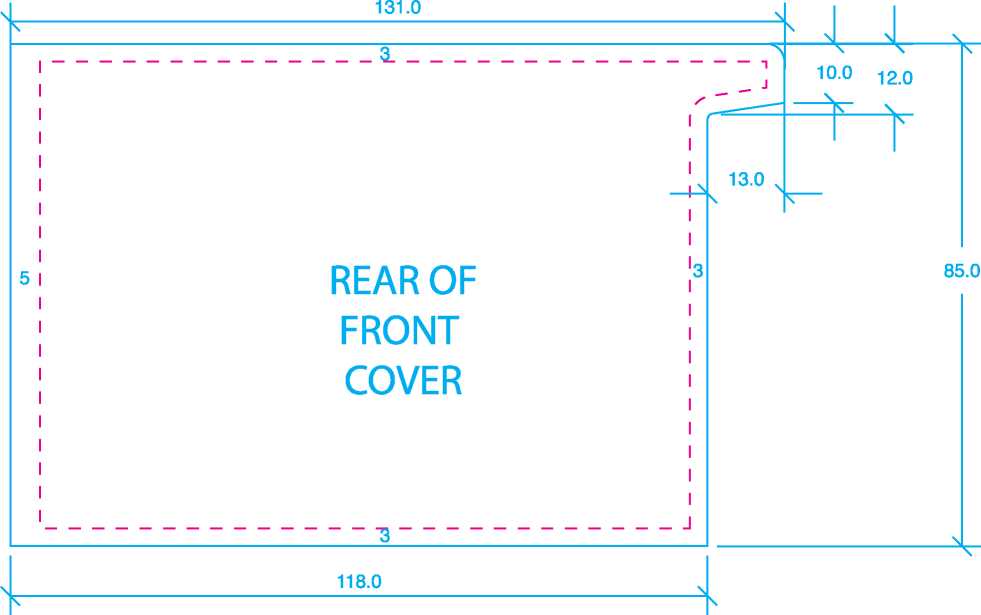
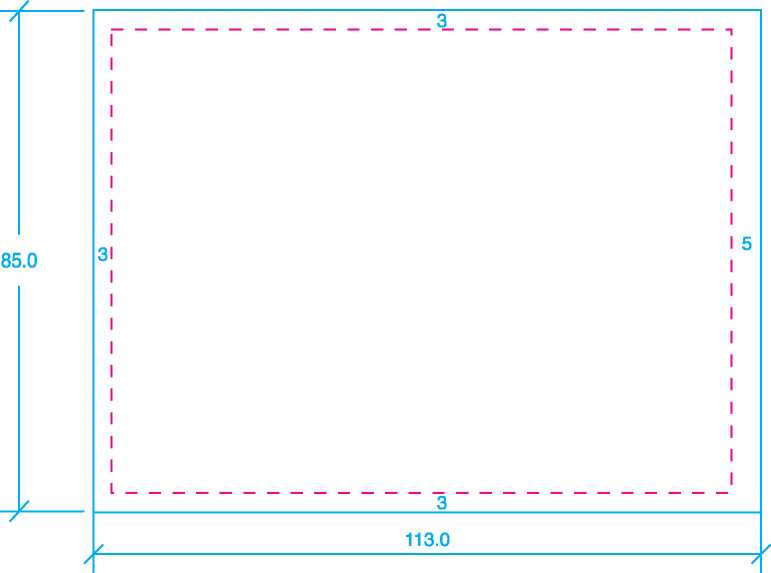
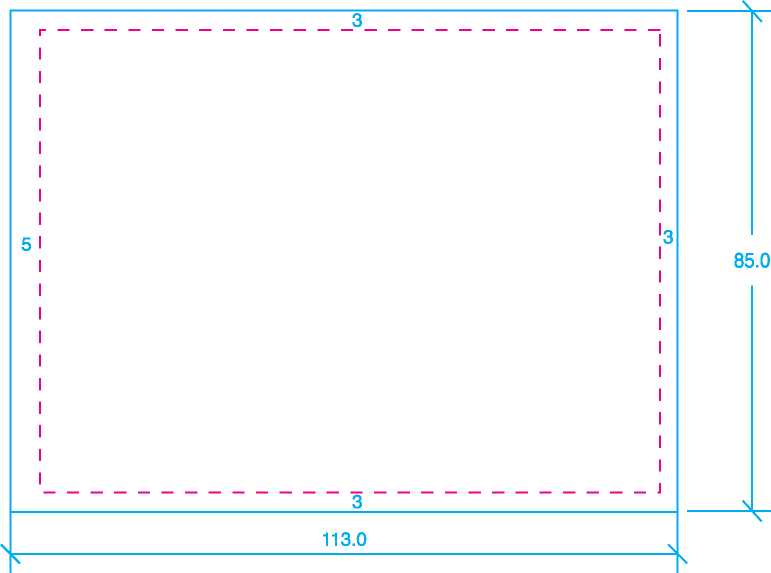
Size: 143mm x 105mm |
Colours: ■ Pantone 647 ■ Pantone 254 ■ Black |
Fonts: Vectora Light & Vectora Bold |
|
PL Number: 41830/0037 |
Date: 6.7.15 |
Version: 1 |
|
Client: NRIM |
Product: Sodium Feredetate 190mg/5ml Oral Solution 500ml |
Strength: 125ml |
--© >
O O O I
T3
_l
- if a child has been taking Sodium feredetate for a long time or at high doses as this can lead to toxic accumulation in the body
- if you are having tests on your stools as iron preparations colour the faeces black and can interfere with test results
Other medicines and Sodium Feredetate
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
This includes any medicines you buy without a prescription, including iron-containing medicines or tonics. This is because Sodium Feredetate can affect the way in which some other medicines work. Also some medicines can affect the way Sodium Feredetate works.
Tell your doctor if you are taking any of the following: dimercaprol (for metal poisoning) iron-containing medicines or tonics
chloramphenicol, fluoroquinolones or tetracyclines (to treat infections) penicillamine (for rheumatoid arthritis) methyldopa (for high blood pressure) mycophenolate (to prevent organ transplant rejection) levodopa, carbidopa or entacapone (for Parkinson's disease) bisphosphonates (for osteoporosis) thyroxine (for thyroid problems) trientine (for Wilson's Disease) cholestyramine (for high cholesterol) proton-pump-inhibitors e.g. omeprazole (for stomach ulcers)
- bicarbonates, carbonates, calcium, magnesium, zinc and other mineral supplements (indigestion and antacid remedies)
It _tBa„coffee, eggsjmiJJi ascorbic acid (vitamin riand citric acidjbese may interfere with-Sodiuimieredetate
Pregnancy and breast-feeding — — — — — — — — — — — — — — — — — —
If you are pregnant, planning to have a baby or breast-feeding, ask your doctor for advice before taking this medicine.
I
Driving and using machines
Sodium Feredetate is not expected to have an effect on your ability to drive or operate machinery.
- methyl hydroxybenzoate (E218), propyl hydroxybenzoate (E216). These ingredients may cause I allergic reactions (possibly delayed).
|- ponceau 4R (E124). This ingredient may cause allergic reactions.
- sorbitol. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product. Sorbitol may also cause mild laxative effects.
- ethanol (alcohol). Sodium Feredetate contains small amounts of ethanol less than 100 mg per 5 ml.
- sodium. Sodium Feredetate contains approximately 11.51 mg of sodium per 5 ml dose. Take this into account if you are on a controlled sodium diet.
I
3. HOW TO TAKE SODIUM FEREDETATE
Always take this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The oral solution is to be taken by mouth.
-7'
8
- Adults (including elderly older than 65 years):
o 1 or 2 x 5 ml spoonful 3 times a day.
- Children:
o 3 to 6 mg of elemental iron per 1 kg of body weight.
o The maximum daily dose is 200 mg of elemental iron* (36.3 ml of Sodium Feredetate), divided into 2 or 3 daily doses.
o To be given by a healthcare professional only.
If you
If you accidentally take too much Sodium Feredetate or give too much to a child, tell your doctor or contact your hospital immediately. Take this leaflet, the container and any remaining oral solution with you.
An overdose may cause:
~s~
~s~
3 - Babies of low birth-weight who are solely breast-fed:
I o 5 mg of elemental iron* (0.9 ml of Sodium Feredetate given in a 1 ml syringe) daily.
I o Depending on the body weight, a higher dose (up to 2 mg per 1 kg of body weight) of elementa
iron* daily may be required for exclusively breast-fed babies. o To be given by a healthcare professional only.
o 1 x 5 ml spoonful daily.
o 1 - 2 x 5 ml spoonful daily.
4. POSSIBLE SIDE EFFECTS
- you to feel sick (nausea)
- you to be sick (vomiting, which may contain blood)
- stomach pains |- diarrhoea
- blood in your stools
- tiredness
- cold and sweaty skin
- fast heart beat
- high blood sugar
|- high blood acidity (metabolic acidosis).
If you forget to take Sodium Feredetate
If you forget a dose, take it as soon as you remember. However, if it is nearly time for the next dose, skip the missed dose. Do NOT take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this
medicine, ask yi
our doctor or pharmacist.
-v
V
10
Like all medicines, Sodium Feredetate can cause side effects, although not everybody gets them.
You may feel sick (nausea) or have mild diarrhoea in the early stages of treatment. These effects should quickly disappear if you stop taking Sodium Feredetate for a short time.
When treatment is restarted, a lower dose of your medicine should be taken. If you are not sure what your dose should be, talk to your doctor.
If your doctor tells you to take Sodium Feredetate at doses higher than is stated in this leaflet, you may experience mild diarrhoea.
If you experience any of the following side effects, STOP taking Sodium Feredetate and see g a doctor or go to a hospital IMMEDIATELY:
- allergic reactions - symptoms may include itchy skin rash, swelling of the face, lips, tongue or throat, or difficulty breathing or swallowing.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme. Website: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE SODIUM FEREDETATE_
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date, which is stated on the bottle label after EXP. The expiry date refers to the last day of the month.
Do not use Sodium Feredetate after the bottle has been opened for more than 3 months.
I
Store in the original package in order to protect from light.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
I
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Sodium Feredetate contains
The active substance is sodium feredetate (also known as sodium iron edetate) 190 mg/5 ml.
The other ingredients are methyl hydroxybenzoate (E218), propyl hydroxybenzoate (E216), citric aci g monohydrate, saccharin sodium, glycerol, sorbitol (E420), ethanol, ponceau 4R (E124), black cheri flavouring and water.
What Sodium Feredetate looks like and contents of the pack
Sodium feredetate is a red coloured liquid. It is supplied in amber-coloured glass bottles with a child resistant cap containing 500ml of oral solution.
Marketing Authorisation Holder and Manufacturer
NRIM Limited, Unit 15 Moorcroft, Harlington Road, Hillingdon, UB8 3HD, United Kingdom.
This leaflet was last revised in 07/2015.
I
11 12
vO
vj
Ln
ISJ
00
o^
r
i
|
II |
N |
II |
|
T3 |
T3 |
n |
|
fD =3 11 |
=j' |
c r+ r+ |
|
r+ CU |
n> | |
|
S' |
C7 | |
|
<x> |
fD |
3 |
|
Cll |
fD |
Q>

|
Client: NRIM |
PL Number: 41830/0037 |
Size: 143mm x 105mm |
|
Product: Sodium Feredetate 190mg/5ml Oral Solution Braille |
Date: 6.7.15 |
Colours: N/A |
|
Strength: 125ml |
Version: 1 |
Fonts: SD Pharma Braille UK |
Arta Creative Solutions Ltd Suite 1, Interlinks House 81c Church Road London, NW4 4DP
020 8203 5976 020 8203 2357 info@artacreative.co.uk www.artacreative.co.uk
creative solutions