Solvent For Losec I.v. Injection 40Mg
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Solvent for Losec* i.v. Injection 40 mg.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each ampoule contains 10ml of solvent for injection.
3 PHARMACEUTICAL FORM
Solvent for solution for injection
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
To be used as a Solvent for i.v. injection with 1 vial of powder for injection which contains Omeprazole sodium Ph. Eur., equivalent to Omeprazole 40mg
4.2 Posology and method of administration
Dosage and Administration
The intravenous solution is obtained by dissolving the lyophilised omeprazole in the solvent provided. No other solvent should be used and the solution must be given by intravenous injection and not added to any other solutions for injection. The injection should be given slowly over a period of 5 minutes. The solution should be used within 4 hours of preparation.
Use in the Elderly:
Dosage adjustment is not necessary.
Use in Children:
There is limited experience of use in children.
4.3 Contraindications
Known hypersensitivity to macrogol (polyethylene glycol) and citric acid monohydrate.
4.4 Special warnings and precautions for use
None stated.
4.5 Interaction with other medicinal products and other forms of interaction
None stated.
4.6 Pregnancy and lactation
None stated.
4.7 Effects on ability to drive and use machines
None stated.
4.8 Undesirable effects
None stated.
4.9 Overdose
None stated.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
None stated.
5.2 Pharmacokinetic properties
Macrogols entering the systemic circulation are predominantly excreted unchanged in the urine, low molecular weight macrogols may be partly metabolised.
5.3 Preclinical safety data
Citric acid and macrogols are commonly used in pharmaceutical preparations, no addition information is necessary.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Macrogol 400, Citric acid monohydrate and water for injections.
6.2 Incompatibilities
None known
6.3 Shelf life
Unopened pack : 2 years.
Reconstituted solution : 4 hours.
6.4 Special precautions for storage
Do not store above 25*C. Keep container in outer carton.
6.5 Nature and contents of container
A clear, Type I (OPC) glass ampoule containing solvent for intravenous administration, packed in a plastic tray with a glass vial in a hard cardboard box.
6.6 Special precautions for disposal
The solvent in the ampoule is used to reconstitute lyophilised powder to provide a solution for i.v. injection.
Use on one patient during one treatment only.
DO NOT USE if any particles are present in the reconstituted solution.
From a microbiological point of view, once opened and reconstituted the product may be stored for a maximum of 4 hours at 25°C. Other in-use storage times and conditions are the responsibility of the user.
Any unused portion should be discarded.
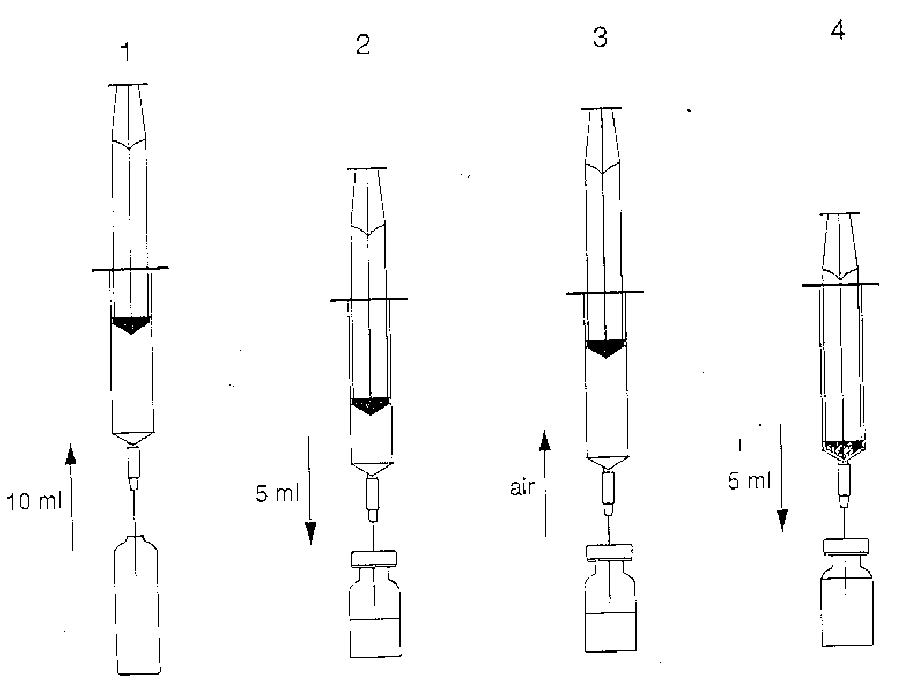
Preparation:
NOTE: Stages 1 to 5 should be done in immediate sequence.
1.With a syringe draw 10ml of solvent from the ampoule.
2. Add approximately 5ml of the solvent to the vial with the lyophilised powder.
3. Withdraw as much air as possible from the vial back into the syringe in order to reduce positive pressure. This will make it easier to add the remaining solvent.
4. Add the remaining solvent into the vial, make sure that the syringe is empty.
5. Rotate and shake the vial to ensure all the lyophilised powder has been dissolved.
(see Diagrams)
7 MARKETING AUTHORISATION HOLDER
AstraZeneca UK Limited 600Capability Green Luton LU13LU United Kingdom
8 MARKETING AUTHORISATION NUMBER(S)
PL 17901/0167
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE
AUTHORISATION
17/11/1998 / 07/06/2006
10 DATE OF REVISION OF THE TEXT
16/01/2014