Spiriva 18 Microgram Inhalation Powder Hard Capsule
Assessed against UK PIL dated August 2005
(APPROVED
[ By austino at 4:35 pm, 1/9/07
LANDMARK PHARMA LTD
PROPOSED TEXT: PATIENT INFORMATION LEAFLET PL (PI) 21828/0137 Page 1 of 2
HANDIHALER Instructions for use For use with:
Spiriva® 18 Microgram Inhalation Powder, Hard Capsules (tiotropium)
The HANDIHALER enables you to inhale the medicine contained in the SPIRIVA INHALATION POWDER capsule - that your physician has prescribed for your breathing problems.
Remember to carefully follow your doctor's instructions for using SPIRIVA INHALATION POWDER. The HANDIHALER is especially designed for SPIRIVA
INHALATION POWDER you must not use it to take any other medication. You can use your HANDIHALER for up to one year to take your medication.

The HANDIHALER
1. dust cap
2. mouthpiece
3. base
4. piercing button
5. centre chamber
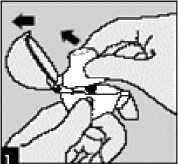
Open the dust cap by pulling it upwards. Then open the mouthpiece.



Remove a SPIRIVA INHALATION POWDER capsule from the blister (only immediately before use) and place it in the centre chamber. Put the SPIRIVA INHAlAtiOn POWDER capsule in the centre chamber (5), as illustrated. It does not matter which way the capsule is placed in the chamber.
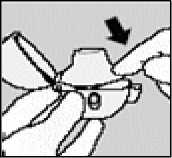
Close the mouthpiece firmly until you hear a click, leaving the dust cap open.
Hold the HANDIHALER with the mouthpiece upwards and press the green button completely in once, and release. This makes holes in the capsule and allows the medication to be released when you breathe in.

Breathe out completely. Important:
Please avoid breathing into the mouthpiece at any time.
Raise the HANDIHALER to your mouth and close your lips tightly around the mouthpiece. Keep your head in an upright position and breathe in slowly and deeply but at a rate sufficient to hear the capsule vibrate. Breathe until your lungs are full; then hold your breath as long as comfortable and at the same time take the HANDIHALER out of your
Original Application Date: 06/12/05
LANDMARK PHARMA LTD
PROPOSED TEXT: PATIENT INFORMATION LEAFLET PL (PI) 21828/0137 Page 2 of 2
mouth. Resume normal breathing. Repeat step 5 and 6 once, this will empty the capsule completely.
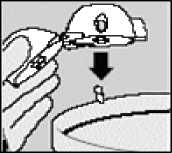
Open the mouthpiece again. Tip out the used capsule and dispose. Close the mouthpiece and dust cap for storage of your HANDIHALER.
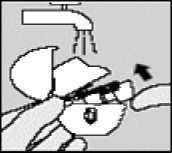
Clean the HANDIHALER once a month. Open the dust cap and mouthpiece. Then open the base by lifting the piercing button. Rinse the complete inhaler with warm water to remove any powder. Dry the HANDIHALER thoroughly by tipping excess of water out on a paper towel and air-dry afterwards, leaving the dust cap, mouthpiece and base open. It takes 24 hours to air dry, so clean it right after you have used it and it will be ready for your next dose. If needed the outside of the mouthpiece may be cleaned with a moist but not wet tissue.
Blister handling
Separate the blister strips by tearing along the perforation.

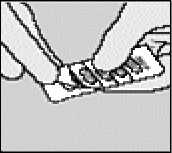
Peel back foil (only immediately before use) using the tab until one capsule is fully visible. In case a second capsule is exposed to air inadvertently this capsule has to be discarded.
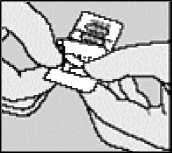
Remove capsule. SPIRIVA INHALATION POWDER capsules contain only a small amount of powder so that the capsule is only partially filled.
SPIRIVA® and HANDIHALER® are trademarks of Boehringer Ingelheim Pharma GmbH & Co. KG, Germany
Leaflet Revision Date: 14 November 2006 Ref: SPI0137-INSTv1
Original Application Date: 06/12/05
Date of Amendment/Printing: 14/11/2006 Name: LANDMARK PHARMA LTD Signed: