Spiriva 18 Microgram Inhalation Powder Hard Capsules
Out of date information, search anotherCommon:
Uncommon:
Rare:
Not known:
PACKAGE LEAFLET: INFORMATION FOR THE USER
Spiriva 18 microgram Inhalation Powder, Hard Capsules
(tiotropium bromide)
Your medicine is known by the above name but will be referred to as Spiriva 18 microgram throughout the following.
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Spiriva 18 microgram is and what it is used for
2. What you need to know before you take Spiriva 18 microgram
3. How to take Spiriva 18 microgram
4. Possible side effects
5. How to store Spiriva 18 microgram
6. Contents of the pack and other information
1. WHAT SPIRIVA 18 MICROGRAM IS AND WHAT IT IS USED FOR
Spiriva 18 microgram helps people who have chronic obstructive pulmonary disease (COPD) to breathe more easily. COPD is a chronic lung disease that causes shortness of breath and coughing. The term COPD is associated with the conditions chronic bronchitis and emphysema. As COPD is a chronic disease you should take Spiriva 18 microgram every day and not only when you have breathing problems or other symptoms of COPD.
Spiriva 18 microgram is a long-acting bronchodilator that helps to open your airways and makes it easier to get air in and out of the lungs. Regular use of Spiriva 18 microgram can also help you when you have on-going shortness of breath related to your disease and will help you to minimise the effects of the disease on your everyday life. It also helps you to be active longer. Daily use of Spiriva 18 microgram will also help to prevent sudden, short-term worsening of your COPD symptoms which may last for several days. The effect of this medicine lasts for 24 hours, so you only need to take it once a day. For correct dosing of Spiriva 18 microgram please see section 3. How to take Spiriva 18 microgram and the instructions for use provided on the other side of the leaflet.
2. WHAT YOU NEED TO KNOW BEFORE YOU TAKE SPIRIVA 18 MICROGRAM
Please read the following questions carefully. If you can answer any of these questions with “Yes” please discuss this with your doctor before taking Spiriva 18 microgram
• are you allergic to tiotropium, atropine or similar drugs such as ipratropium or oxitropium or to milk protein?
• are you taking any other medicinal products containing ipratropium or oxitropium?
• are you pregnant, do you think you are pregnant, or are you breast-feeding?
• are you suffering from narrow angle glaucoma, prostate problems or have difficulty passing urine?
• do you have any kidney problems?
• have you suffered from a myocardial infarction during the last 6 months or from any unstable or life threatening irregular heart beat or severe heart failure within the past year?
Do not take Spiriva 18 microgram
You should not use Spiriva 18 microgram if you are allergic (hypersensitive) to tiotropium, its active ingredient or to lactose monohydrate which contains milk protein.
You should also not use Spiriva 18 microgram if you are allergic (hypersensitive) to atropine or substances related to it, e.g. ipratropium or oxitropium.
Warnings and precautions
• Talk to your doctor if you suffer from narrow angle glaucoma, prostate problems or have difficulty passing urine.
• If you have problems with your kidneys, please consult your doctor.
• Spiriva 18 microgram is indicated for maintenance treatment of your chronic obstructive pulmonary disease, it should not be used to treat a sudden attack of breathlessness or wheezing.
• Immediate allergic reactions such as rash, swelling, itching, wheezing or breathlessness may occur after administration of Spiriva 18 microgram. If this occurs, please consult your doctor immediately.
• Inhaled medicines such as Spiriva 18 microgram may cause tightness of the chest, coughing, wheezing or breathlessness immediately after inhalation. If this occurs, please consult your doctor immediately.
• Take care not to let the inhalation powder enter your eye as this may result in precipitation or worsening of narrow-angle glaucoma, which is a disease of the eyes. Eye pain or discomfort, blurred vision, seeing halos around lights or coloured images in association with red eyes may be signs of an acute attack of narrow-angle glaucoma. Eye symptoms may be accompanied by headache, nausea or vomiting. You should stop using tiotropium bromide and immediately consult your doctor, preferably an eye specialist, when signs and symptoms of narrow-angle glaucoma appear.
• Dry mouth, which has been observed with anti-cholinergic treatment, may in the long term be associated with dental caries. Therefore, please remember to pay attention to oral hygiene.
• In case you have suffered from a myocardial infarction during the last 6 months or from any unstable or life threatening irregular heart beat or severe heart failure within the past year, please, inform your doctor. This is important to decide if Spiriva is the right medicine for you to take.
• Do not take Spiriva 18 microgram more frequently than once daily.
Children and adolescents
Spiriva 18 microgram is not recommended for children and adolescents under 18 years.
Other medicines and Spiriva 18 microgram
Please inform your doctor or pharmacist if you are taking, or have recently taken, any other medicines, even those not prescribed.
Please tell your doctor or pharmacist if you are taking/have taken similar medicines for your lung disease, such as ipratropium or oxitropium.
No specific side effects have been reported when Spiriva 18 microgram has been taken together with other products used to treat COPD such as reliever inhalers, e.g. salbutamol, methylxanthines, e.g. theophylline and/or oral and inhaled steroids e.g. prednisolone.
Pregnancy and breast-feeding
If you are pregnant or believe you are pregnant, or if you are breast-feeding, consult with your doctor. You should not use this medicine unless specifically recommended by your doctor.
Driving and using machines
The occurrence of dizziness, blurred vision, or headache may influence the ability to drive and use machinery.
Spiriva 18 microgram contains lactose monohydrate
When taken according to dosage recommendations, one capsule once a day, each dose supplies up to 5.5mg lactose monohydrate.
3. HOW TO TAKE SPIRIVA 18 MICROGRAM
Follow your doctor’s instructions about when and how to take your medicine. If you are unsure ask your doctor or pharmacist.
The recommended dose is to inhale the contents of 1 capsule (18 micrograms of tiotropium) once a day. Do not take more than the recommended dose.
Spiriva 18 microgram is not recommended for children and adolescents under 18 years.
You should try to take the capsule at the same time every day. This is important because Spiriva 18 microgram is effective over 24 hours.
The capsules are only for inhalation and not for oral intake.
Do not swallow the capsules.
The HandiHaler device, which you should put the Spiriva 18 microgram capsule into, makes holes in the capsule and allows you to breathe in the powder.
Make sure that you have a HandiHaler and that you can use it properly. The instructions for use of the HandiHaler are provided on the other side of this leaflet.
Make sure that you do not blow into the HandiHaler.
If you have any problems using the HandiHaler, ask your doctor, nurse or pharmacist to show you how it works.
You should clean your HandiHaler once a month. Cleaning instructions for the HandiHaler are provided on the other side of this leaflet.
When taking Spiriva 18 microgram, take care not to let any of the powder enter your eyes. If any powder does get into your eyes you may get blurred vision, eye pain and/or red eyes, you should wash your eyes in warm water immediately. Then talk to your doctor immediately for further advice.
If you feel that your breathing is worsening, you should tell your doctor as soon as possible.
If you take more Spiriva 18 microgram than you should
If you inhale from more than 1 capsule of Spiriva 18 microgram in a day, you should talk to your doctor immediately. You may be at a higher risk of experiencing a side effect such as dry mouth, constipation, difficulties passing urine, increased heart beat, or blurred vision.
If you forget to take Spiriva 18 microgram
If you forget to take a dose, take one as soon as you remember but do not take two doses at the same time or on the same day. Then take your next dose as usual.
If you stop taking Spiriva 18 microgram
Before you stop taking Spiriva 18 microgram, you should talk to your doctor or your pharmacist. If you stop taking Spiriva 18 microgram the signs and symptoms of COPD may worsen.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them. Evaluation of the side effects is based on the following frequencies:
may affect up to 1 in 10 people
may affect up to 1 in 100 people
may affect up to 1 in 1,000 people
frequency cannot be estimated from the available data
The side effects described below have been experienced by people taking this medicine and they are listed according to frequency as either common, uncommon, rare or not known.
Common:
• Dry mouth: this is usually mild
Uncommon:
• Dizziness
• Headache
• Taste disorders
• Blurred vision
• Irregular heart beat (atrial fibrillation)
• Sore throat (pharyngitis)
• Hoarseness (dysphonia)
• Cough
• Heart burn (gastrooesophageal reflux disease)
• Constipation
• Fungal infections of the oral cavity and throat (oropharyngeal candidiasis)
• Rash
• Difficulty passing urine (urinary retention)
• Painful urination (dysuria)
Rare:
• Difficulty in sleeping (insomnia)
• Seeing halos around lights or coloured images in association with red eyes (glaucoma)
• Increase of the measured eye pressure
• Irregular heart beat (supraventricular tachycardia)
• Faster heart beat (tachycardia)
• Feeling your heartbeat (palpitations)
• Tightness of the chest, associated with coughing, wheezing or breathlessness immediately after inhalation (bronchospasm)
• Nosebleed (epistaxis)
• Inflammation of the larynx (laryngitis)
• Inflammation of the sinuses (sinusitis)
• Blockage of intestines or absence of bowel movements (intestinal obstruction including ileus paralytic)
• Inflammation of the gums (gingivitis)
• Inflammation of the tongue (glossitis)
• Difficulties swallowing (dysphagia)
• Inflammation of the mouth (stomatitis)
• Feeling sick (nausea)
• Allergic reactions (hypersensitivity), including immediate reactions
• Serious allergic reaction which causes swelling of the face or throat (angioedema)
• Nettle rash (urticaria)
• Itching (pruritus)
• Infections of the urinary tract
Not known:
• Depletion of body water (dehydration)
• Tooth decay (dental caries)
• Severe allergic reaction (anaphylactic reaction)
• Infections or ulcerations of the skin
• Dryness of the skin
• Swelling of joints
Serious side effects include allergic reactions which cause swelling of the face or throat (angioedema) or other hypersensitivity reactions (such as sudden reduction of your blood pressure or dizziness) may occur individually or as part of severe allergic reaction (anaphylactic reaction) after administration of Spiriva 18 microgram. In addition, in common with all inhaled medicines, some patients may experience an unexpected tightness of the chest, coughing, wheezing or breathlessness immediately after inhalation (bronchospasm). If any of these occur, please consult your doctor immediately.
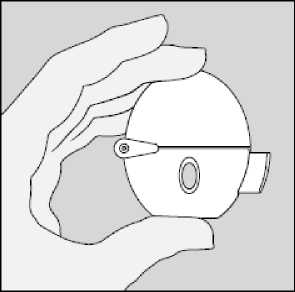
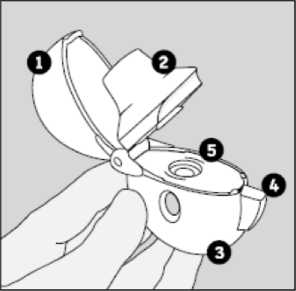
The HandiHaler
K Dust cap ® Mouthpiece ^) Base [7) Piercing button [Y) Centre chamber
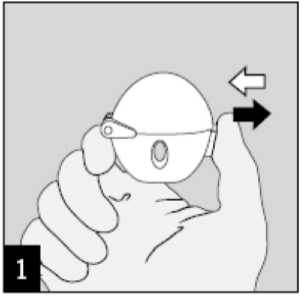
To release the dust cap press the piercing button completely in and let go.
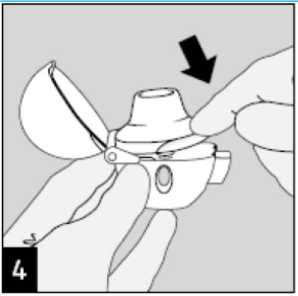
Close the mouthpiece firmly until you hear a click, leaving the dust cap open.
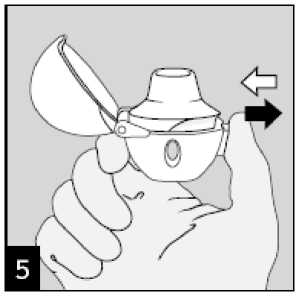
Breathe out completely.
Important: Please avoid breathing into the mouthpiece at any time.
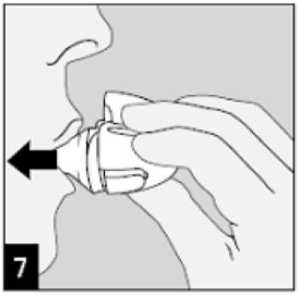
Open the mouthpiece again. Tip out the used capsule and dispose. Close the mouthpiece and dust cap for storage of your HandiHaler device.
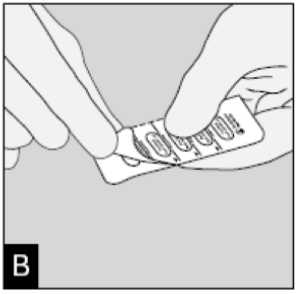
Separate the blister strips by tearing along the perforation.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE SPIRIVA 18 MICROGRAM
Keep out of the sight and reach of children.
Do not use Spiriva 18 microgram after the expiry date shown on the blister foil and carton. The expiry date refers to the last day of that month.
Once you have taken your first capsule of Spiriva 18 microgram from the blister strip you must continue to take the capsules for the next 9 days, one capsule a day, from the same strip.
Do not store above 25 °C. Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Only keep the medicine if your doctor tells you to.
If the medicine becomes discoloured or show signs of any deterioration, you should seek the advice of your pharmacist.
6. CONTENTS OF THE PACK AND OTHER INFORMATION What Spiriva 18 microgram contains
Each capsule contains 18 micrograms of the active ingredient tiotropium (as bromide monohydrate).
The capsule also contains the inactive ingredient lactose monohydrate.
What Spiriva 18 microgram looks like and contents of the pack
The capsule shell contains gelatin and black printing ink. Your medicine is a light green, hard capsule, containing a white or yellowish powder, with the product code (TI 01) and company logo printed on the capsule in black ink, supplied with a plastic grey inhaler device with light green piercing button marked ‘Handihaler’ and Boehringer Ingelheim and a logo.
This product is available in blister packs of 30 capsules, supplied with 1 inhaler device, known as the HandiHaler device.
Spiriva 18 microgram and the HANDIHALER are manufactured by Boehringer Ingelheim Pharma GmbH & Co. KG, Binger Strasse 173, D-55216 Ingelheim am Rhein, Germany and are procured from within the EU by Product Licence holder: Caseview (PL) Limited, 20 Alliance Court, Alliance Road, London W3 0RB and repackaged by OPD Laboratories Ltd, Watford, Herts WD24 4PR.
PL 13826/1214 |POM|
Spiriva 18 microgram Inhalation Powder, Hard Capsules Leaflet revision date (ref) 18/02/2015
To request a copy of this leaflet in Braille, large print or audio please call 01923 332 796.
HandiHaler Instructions for use
Dear Patient,
The HandiHaler enables you to inhale the medicine contained in the Spiriva 18 microgram capsule - that your physician has prescribed for your breathing problems.
Remember to carefully follow your doctor’s instructions for using Spiriva 18 microgram. The HandiHaler is especially designed for Spiriva 18 microgram. You must not use it to take any other medication. You can use your HandiHaler for up to one year to take your medication.
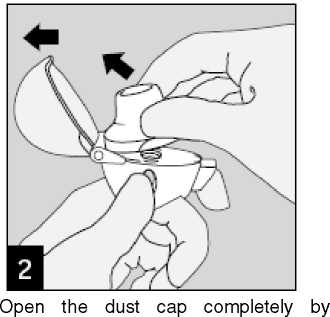
pulling it upwards.
Then open the mouthpiece by pulling it upwards.
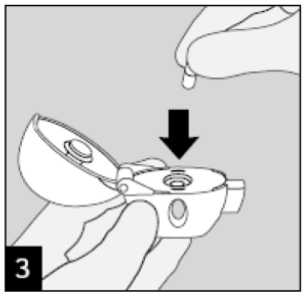
Remove a Spiriva 18 microgram capsule from the blister (only immediately before use, see blister handling) and place it in the centre
chamber ( ), as illustrated. It does not
matter which way the capsule is placed in the chamber.
Hold the HandiHaler device with the mouthpiece upwards and press the piercing button completely in only once, and release. This makes holes in the capsule and allows the medication to be released when you breathe in.
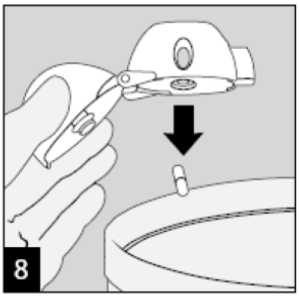
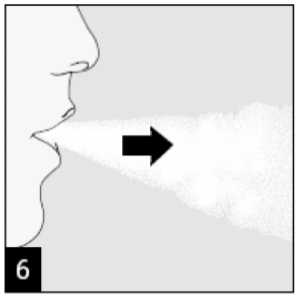
Raise the HandiHaler to your mouth and close your lips tightly around the mouthpiece. Keep your head in an upright position and breathe in slowly and deeply but at a rate sufficient to hear or feel the capsule vibrate. Breathe until your lungs are full; then hold your breath as long as comfortable and at the same time take the HandiHaler out of your mouth. Resume normal breathing. Repeat steps 6 and 7 once, in order to empty the capsule completely.
Cleaning your HandiHaler:
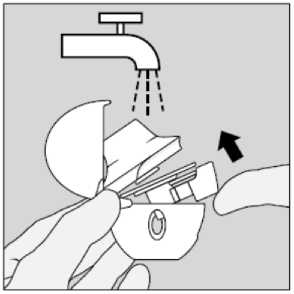
Clean the HandiHaler once a month. Open the dust cap and mouthpiece. Then open the base by lifting the piercing button. Rinse the complete inhaler with warm water to remove any powder. Dry the HandiHaler thoroughly by tipping excess of water out on a paper towel and air-dry afterwards, leaving the dust cap, mouthpiece and base open. It takes 24 hours to air dry, so clean it right after you have used it and it will be ready for your next dose. If needed the outside of the mouthpiece may be cleaned with a moist but not wet tissue.
Blister handling
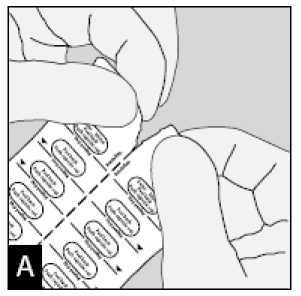
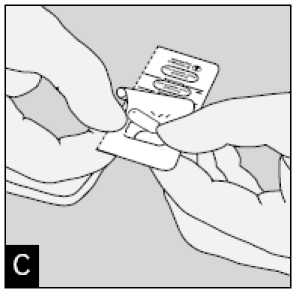
Peel back foil (only immediately before use) using the tab until one capsule is fully visible.
In case a second capsule is exposed to air inadvertently this capsule has to be discarded.
Remove capsule.
Spiriva 18 microgram capsules contain only a small amount of powder so that the capsule is only partially filled.
‘Spiriva’ and ‘HandiHaler’ are registered trademarks of Boehringer Ingelheim Pharma GmbH & Co. KG.