Spiriva Respimat Inhaler
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. ‘See section 4’
Package leaflet: Information for the user
Spiriva® Respimat® inhaler
(tiotropium bromide)
The name of your medicine is Spiriva Respimat inhaler but will be referred to as Spiriva Respimat throughout this leaflet.
What is in this leaflet
1. What Spiriva Respimat is and what it is used for
2. What you need to know before you take Spiriva Respimat
3. How to take Spiriva Respimat
4. Possible side effects
5. Howto store Spiriva Respimat
6. Contents of the pack and other information
1. What Spiriva Respimat is and what it is used for
Spiriva Respimat helps people who have chronic obstructive pulmonary disease (COPD) or asthma to breathe more easily. COPD is a long-term lung disease that causes shortness of breath and coughing. The term COPD is associated with the conditions chronic bronchitis and emphysema. Asthma is a long-term disease characterised by airway inflammation and narrowing of the airways. As COPD and asthma are long-term diseases you should take Spiriva Respimat every day and not only when you have breathing problems or other symptoms. When used to treat asthma you should use Spiriva Respimat in addition to so-called inhaled corticosteroids and long-acting S2 agonists.
Spiriva Respimat is a long-acting bronchodilatorthat helps to open your airways and makes it easier to get air in and out of the lungs. Regular use of Spiriva Respimat can also help you when you have on-going shortness of breath related to your disease, and will help to minimise the effects of the disease on your everyday life. Daily use of Spiriva Respimat will also help to prevent any sudden, short-term worsening of your COPD symptoms which may last for several days.
For correct dosing of Spiriva Respimat please see section 3. ‘Howto take Spiriva Respimat’ and the instructions for use provided on the other side of the leaflet.
2. What you need to know before you take Spiriva Respimat
Please read the following questions carefully. If you
can answer any of these questions with ‘Yes’ please discuss this with your doctor before taking Spiriva Respimat.
- are you allergic (hypersensitive) to tiotropium, atropine or similar drugs such as ipratropium or oxitropium?
- are you taking any other medicinal products containing ipratropium or oxitropium?
- are you pregnant, do you think you are pregnant, or are you breast-feeding?
- are you suffering from blurred vision, eye pain and/ or red eyes, prostate problems or have difficulty passing urine?
- do you have any kidney problems?
- have you suffered from a myocardial infarction during the last 6 months or from any unstable or life-threatening irregular heart beat or severe heart failure within the past year?
Do not use Spiriva Respimat
- if you are allergic (hypersensitive) to tiotropium, its active ingredient or any of the other ingredients of this medicine (listed in section 6)
- if you are allergic (hypersensitive) to atropine or substances related to it, e.g. ipratropium or oxitropium
Warnings and precautions
Talk to your doctor before taking Spiriva Respimat.
When taking Spiriva Respimat take care not to let any spray enter your eyes. This may result in eye pain or discomfort, blurred vision, seeing halos around lights or coloured images in association with red eyes (i.e. narrow angle glaucoma). Eye symptoms may be accompanied by headache, nausea or vomiting. Wash your eyes in warm water, stop using tiotropium bromide and immediately consult your doctor for further advice.
If your breathing has got worse or if you experience rash, swelling or itching directly after using your inhaler, stop using it and tell your doctor immediately.
Dry mouth which has been observed with anticholinergic treatment may in the long-term be associated with dental caries. Therefore, please remember to pay attention to oral hygiene.
Spiriva Respimat is indicated for the maintenance treatment of your chronic obstructive pulmonary disease or asthma. Do not use this medicine to treat a sudden attack of breathlessness or wheezing. Your doctor should have given you another inhaler (‘rescue medication’) for this.
Please follow the instructions your doctor has given you.
If you have been prescribed Spiriva Respimat for your asthma it should be added on to inhaled corticosteroids and long-acting S2 agonists. Continue taking the inhaled corticosteroids as prescribed by your doctor, even if you feel better.
In case you have suffered from a myocardial infarction during the last 6 months or from any unstable or life-threatening irregular heart beat or severe heart failure within the past year, please, inform your doctor. This is important to decide if Spiriva Respimat is the right medicine for you to take.
Do not take Spiriva Respimat more frequently than once daily.
You should also contact your doctor if you feel that your breathing is worsening.
If you have cystic fibrosis, tell your doctor because Spiriva Respimat could make your cystic fibrosis symptoms worse.
Children and adolescents
Spiriva Respimat is not recommended for children and adolescents under 18 years.
Other medicines and Spiriva Respimat
Please tell your doctor or pharmacist if you are taking, or have recently taken, any other medicines, including medicines obtained without a prescription.
In particular, please tell your doctor or pharmacist if you are taking/have taken anticholinergic drugs, e.g. ipratropium or oxitropium.
No interaction side effects have been reported when Spiriva Respimat has been taken with other products used to treat COPD such as reliever inhalers (e.g. salbutamol), methylxanthines (e.g. theophylline), antihistamines, mucolytics (e.g. ambroxol), leukotriene modifiers (e.g. montelukast), cromones, anti-lgE treatment (e.g. omalizumab) and/or inhaled or oral steroids (e.g. budesonide, prednisolone).
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. You should not use this medicine unless specifically recommended by your doctor.
Driving and using machines
No studies on the effects and the ability to drive and use machines have been performed. In case dizziness or blurred vision occurs the ability to drive and use machinery may be influenced.
3. How to take Spiriva Respimat
Always take this medicine exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Spiriva Respimat is for inhalation use only.
The recommended dose for adults is:
Spiriva Respimat is effective for 24 hours so you will need to use Spiriva Respimat only ONCE A DAY, if possible at the same time of the day. Each time you use it take TWO PUFFS.
As COPD and asthma are long-term diseases take Spiriva Respimat every day and not only when you experience breathing problems. Do not take more than the recommended dose.
Spiriva Respimat is not recommended for use in children and adolescents below 18 years due to lack of data on safety and efficacy.
Make sure that you know how to use your Spiriva Respimat inhaler properly. The instructions for use of the Spiriva Respimat inhaler are provided on the other side of this leaflet.
If you take more Spiriva Respimat than you should
If you take more Spiriva Respimat than two puffs in one day talk to your doctor immediately. You may be at a higher risk of experiencing a side effect such as dry mouth, constipation, difficulties passing urine, increased heart beat or blurred vision.
If you forget to take Spiriva Respimat
If you forget to take your daily dose (TWO PUFFS ONCE A DAY), don’t worry. Take it as soon as you remember but do not take two doses at the same time or on the same day. Then take your next dose as usual.
If you stop taking Spiriva Respimat
Before you stop taking Spiriva Respimat, you should talk to your doctor or your pharmacist. If you stop taking Spiriva Respimat the signs and symptoms of COPD may worsen.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Evaluation of the side effects is based on the following frequencies:
Common: may affect up to 1 in 10 people
Uncommon: may affect up to 1 in 100 people
Rare: may affect up to 1 in 1,000 people
Not known: frequency cannot be estimated from
the available data
The side effects described below have been experienced by people taking this medicine and they are listed according to frequency as either common, uncommon, rare or not known.
|
Side effect |
Frequency COPD |
Frequency Asthma |
|
Dry mouth: this is usually mild |
Common |
Common |
|
Dizziness |
Uncommon |
Uncommon |
|
Headache |
Uncommon |
Uncommon |
|
Difficulty in sleeping (insomnia) |
Rare |
Uncommon |
|
Irregular heart beat (atrial fibrillation, supraventricular tachycardia) |
Rare |
Not known |
|
Feeling your heart beat (palpitations) |
Rare |
Uncommon |
|
Faster heart beat (tachycardia) |
Rare |
Not known |
|
Cough |
Uncommon |
Uncommon |
|
Nosebleed (epistaxis) |
Rare |
Not known |
|
Inflammation of the throat (pharyngitis) |
Uncommon |
Uncommon |
|
Hoarseness (dysphonia) |
Uncommon |
Uncommon |
|
Tightness of the chest, associated with coughing, wheezing or breathlessness immediately after inhalation (bronchospasm) |
Rare |
Uncommon |
|
Constipation |
Uncommon |
Rare |
|
Fungal infections of the oral cavity and throat (oropharyngeal candidiasis) |
Uncommon |
Uncommon |
|
Difficulties swallowing (dysphagia) |
Rare |
Not known |
|
Rash |
Uncommon |
Rare |
|
Itching (pruritus) |
Uncommon |
Rare |
|
Difficulties passing urine (urinary retention) |
Uncommon |
Not known |
|
Painful urination (dysuria) |
Uncommon |
Not known |
|
Seeing halos around lights or coloured images in association with red eyes (glaucoma) |
Rare |
Not known |
|
Increase of the measured eye pressure |
Rare |
Not known |
|
Blurred vision |
Rare |
Not known |
|
Inflammation ofthe larynx (laryngitis) |
Rare |
Not known |
|
Heartburn (gastrooesophageal reflux disease) |
Rare |
Not known |
|
Dental caries |
Rare |
Not known |
|
Inflammation ofthe gums (gingivitis) |
Rare |
Rare |
|
Inflammation ofthe tongue (glossitis) |
Rare |
Not known |
|
Inflammation ofthe mouth (stomatitis) |
Not known |
Rare |
|
Serious allergic reaction which causes swelling of the mouth and face or throat (angioneurotic oedema) |
Rare |
Rare |
|
Nettle rash (urticaria) |
Rare |
Rare |
|
Infections or ulcerations of the skin |
Rare |
Not known |
|
Dryness ofthe skin |
Rare |
Not known |
|
Hypersensitivity, including immediate reactions |
Not known |
Rare |
|
Infections ofthe urinary tract |
Rare |
Not known |
|
Depletion of body water (dehydration) |
Not known |
Not known |
|
Inflammation in sinuses (sinusitis) |
Not known |
Not known |
|
Blockage of intestines or absence of bowel movements (intestinal obstruction, including ileus paralytic) |
Not known |
Not known |
|
Feeling sick (nausea) |
Not known |
Not known |
|
Severe allergic reaction (anaphylactic reaction) |
Not known |
Not known |
|
Swelling of joint |
Not known |
Not known |
Immediate allergic reactions such as rash, nettle rash (urticaria), swelling of the mouth and face or sudden difficulties in breathing (angioneurotic oedema) or other hypersensitivity reactions (such as sudden reduction of your blood pressure or dizziness) may occur individually or as part of severe allergic reaction (anaphylactic reaction) after administration of Spiriva Respimat. If any of these occur, please consult your doctor immediately.
In addition, in common with all inhaled medicines, some patients may experience an unexpected tightness of the chest, coughing, wheezing or breathlessness immediately after inhalation (bronchospasm).
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.aov.uk/vellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Spiriva Respimat
Keep out of the sight and reach of children.
Do not use Spiriva Respimat after the expiry date which is stated on the carton and on the inhaler label after ‘Exp’. The expiry date refers to the last day of that month.
The inhaler should be discarded at the latest 3 months after first use (see ‘Instructions for Use’ overleaf).
Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist howto dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information
What Spiriva Respimat contains
The active substance is tiotropium. One metered dose contains 2.5 micrograms tiotropium (per treatment dose comprising two spraying), which corresponds to 3,124 micrograms of tiotropium bromide monohydrate. Metered dose is that dose which after passing through the mouthpiece of the inhaler is for the patient.
The other ingredients are:
benzalkonium chloride, disodium edetate, purified
water, and hydrochloric acid 3.6% for pH adjustment.
What Spiriva Respimat looks like and contents of the pack
Spiriva Respimat consists of a single cartridge of the solution for inhalation and one Respimat inhaler. The contribution must be placed in the inhaler before first use.
Single Pack: 1 Respimat inhaler and
1 cartridge, providing 60 puffs (30 medicinal doses)
Manufactured by: Boehringer Ingelheim Pharma GmbH & Co. KG, Binger Strasse 173, D-55216 Ingelheim am Rhein, Germany.
Procured from within the EU and repackaged by the Product Licence holder: B&S Healthcare, Unit 4, Bradfield Road, Ruislip, Middlesex, HA4 ONU, UK.
Spiriva® Respimat® inhaler; PL 18799/2854
I POM I
Leaflet date: 06.05.2016
Instructions for Use
Spiriva® Res pi mat® inhaler
Instructions for use
Introduction
Spiriva Respimat (tiotropium bromide). Read these Instructions for Use before you start using Spiriva Respimat.
You will need to use this inhaler only ONCE A DAY. Each time you use it take TWO PUFFS.
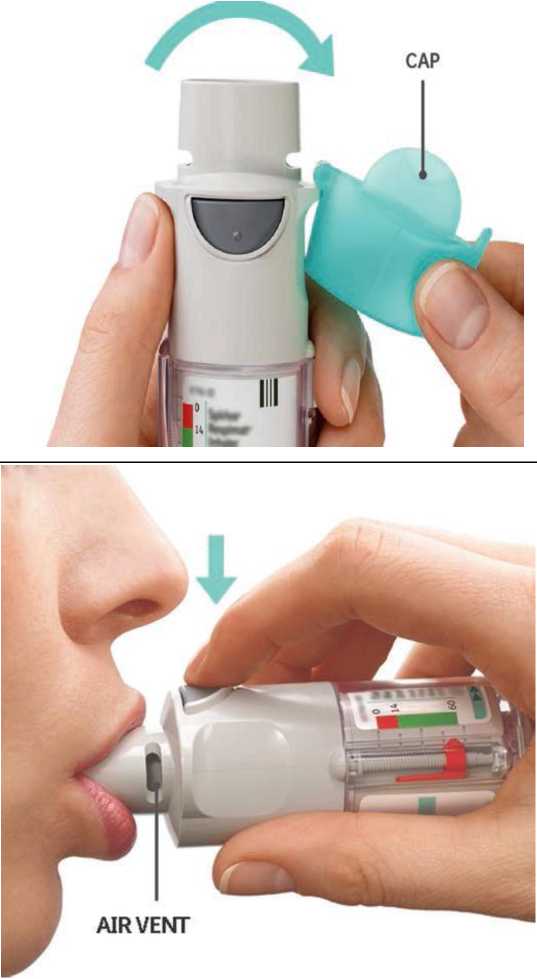
MOUTHPIECE AIR VENT
DOSE-
RELEASE
BUTTON

How to care for your Spiriva Respimat
Clean the mouthpiece including the metal part inside the mouthpiece with a damp cloth or tissue only, at least once a week.
Any minor discolouration in the mouthpiece does not affect your Spiriva Respimat inhaler performance. If necessary, wipe the outside of your Spiriva Respimat inhaler with a damp cloth.
When to get a new Spiriva Respimat
SAFETY CATCH
CLEAR BASE PIERCING ELEMENT

If Spiriva Respimat has not been used for more than 7 days release one puff towards the ground.
If Spiriva Respimat has not been used for more than 21 days repeat steps 4 to 6 under ‘Prepare for first Use’ until a cloud is visible. Then repeat steps 4 to 6 three more times.
Do not touch the piercing element inside the clear base.
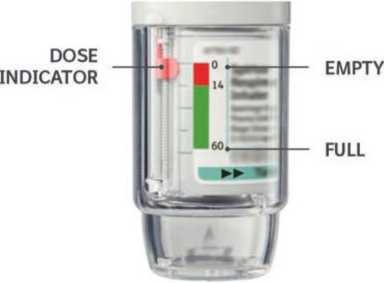
Your Spiriva Respimat inhaler contains 60 puffs (30 doses) if used as indicated (two puffs/ once daily). The dose indicator shows approximately how much medication is left.
When the dose indicator enters the red area of the scale you need to get a new prescription; there is approximately medication for 7 days left (14 puffs). Once the dose indicator reaches the end of the red scale, your Spiriva Respimat locks automatically - no more doses can be released. At this point, the clear base cannot be turned any further.
Spiriva Respimat should be discarded three months after you have prepared it for first use, even if it has not been fully used or used at all.
Prepare for first use

Insert cartridge
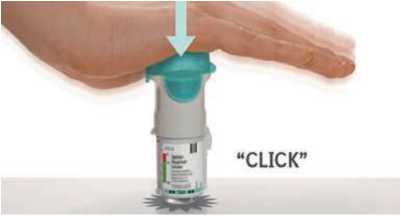
• Insert the narrow end of the cartridge into the inhaler.
• Place the inhaler on a firm surface and push down firmly until it clicks into place.


CLEAR BASE
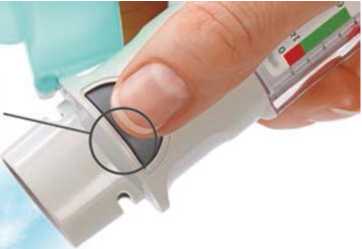
Turn
• Keep the cap closed.
• Turn the clear base in the direction of the arrows on the label until it clicks (half a turn).
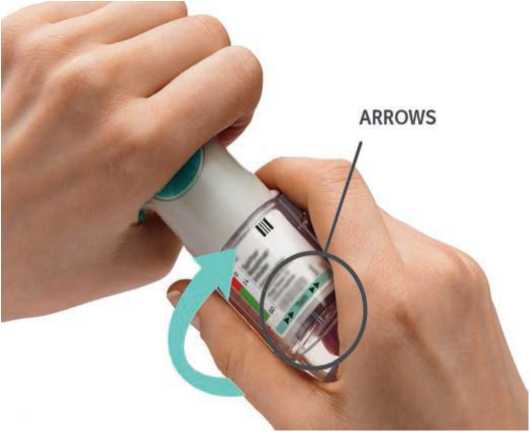
ARROWS

Open
• Open the cap until it snaps fully open.

CAP


Press
• Point the inhaler toward the ground.
• Press the dose-release button
• Close the cap.
• Repeat steps 4-6 until a cloud is visible.
• After a cloud is visible, repeat steps 4-6 three more times.
Your inhaler is now ready to use. These steps will not affect the number of doses available. After preparation your inhaler will be able to deliver 60 puffs (30 doses).
DOSE
RELEASE
BUTTON
Daily use
TURN
• Keep the cap closed.
• TURN the clear base in the direction of the arrows on the label until it Clicks (half a turn).
OPEN
• OPEN the cap until it snaps fully open.
PRESS
• Breathe out slowly and fully.
• Close your lips around the mouthpiece without covering the air vents. Point your Inhaler to the back of your throat.
• While taking a slow, deep breath through your mouth, PRESS the dose-release button and continue to breathe in slowly for as long as comfortable.
• Hold your breath for 10 seconds or for as long as comfortable.
• Repeat TURN, OPEN, PRESS for a total of 2 puffs.
• Close the cap until you use your inhaler again.
It is difficult to insert the cartridge deep enough.
Did you accidentally turn the clear base before inserting the cartridge?
Open the cap, press the dose-release button, then insert the cartridge.
Did you insert the cartridge with the wide end first? Insert the cartridge with the narrow end first.
The dose indicator on the Spiriva Respimat reaches zero too soon.
Did you use Spiriva Respimat as indicated (two puffs/Once daily)?
Spiriva Respimat will last 30 days if used at two puffs once daily.
Did you turn the clear base before you inserted the cartridge? The dose indicator counts each turn of the clear base regardless whether a cartridge has been inserted or not.
Did you spray in the air often to check whether the Spiriva Respimat is working? Once you have prepared Spiriva Respimat, no test-spraying is required if used daily.
Did you insert the cartridge into a used Spiriva Respimat? Always insert a new cartridge into a NEW Spiriva Respimat.
I cannot press the dose-release button.
Did you turn the clear base? If not, turn the clear base in a continuous movement until it clicks (half a turn).
Is the dose indicator on the Spiriva Respimat pointing to zero? The
Spiriva Respimat inhaler is locked after 60 puffs (30 medicinal doses). Prepare and use your new Spiriva Respimat inhaler.
My Spiriva Respimat sprays automatically.
Was the cap open when you turned the clear base? Close the cap, then turn the clear base.
Did you press the dose-release button when turning the clear base?
Close the cap, so the dose-release button is covered, then turn the clear base.
Did you stop when turning the clear base before it clicked? Turn the clear base in a continuous movement until it clicks (half a turn).
I cannot turn the clear base.
Did you turn the clear base already? If the clear base has already been turned, follow steps ‘OPEN’ and ‘PRESS’ under‘Daily Use’ to get your medicine.
Is the dose indicator on the Spiriva Respimat pointing to zero? The Spiriva Respimat inhaler is locked after 60 puffs (30 medicinal doses). Prepare and use your new Spiriva Respimat inhaler.
My Spiriva Respimat doesn’t spray.
Did you insert a cartridge? If not, insert a cartridge.
Did you repeat TURN, OPEN, PRESS less than three times after inserting the cartridge?
Repeat TURN, OPEN, PRESS three times after inserting the cartridge as shown in the steps 4 to 6 under ‘Prepare for first Use’.
Is the dose indicator on the Spiriva Respimat pointing to 0?
If the dose indicator points to 0, you have used up all your medication and the inhaler is locked.
Once your Spiriva Respimat is assembled, do not remove the clear base or the cartridge. Always insert a new cartridge into a NEW Spiriva Respimat.