Sturiban 0.1Mg/Ml Eye Drops Solution
Package leaflet: Information for the user
Sturiban 0.1mg/ml Eye Drops Solution
bimatoprost
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
- The full name of this medicine is Sturiban 0.1mg/ml Eye Drops Solution but within the leaflet it will be referred to as Sturiban.
What is in this leaflet
1. What Sturiban is and what it is used for
2. What you need to know before you use Sturiban
3. How to use Sturiban
4. Possible side effects
5. How to store Sturiban
6. Contents of the pack and other information
1. What Sturiban is and what it is used for
Sturiban is an antiglaucoma preparation. It belongs to a group of medicines called prostamides.
Sturiban eye drops are used to reduce high pressure in the eye. This medicine may be used on its own or with other drops called beta-blockers which also reduce pressure.
Your eye contains a clear, watery liquid that feeds the inside of the eye. Liquid is constantly being drained out of the eye and new liquid is made to replace this. If the liquid cannot drain out quickly enough, the pressure inside the eye builds up. This medicine works by increasing the amount of liquid that is drained. This reduces the pressure inside the eye. If the high pressure is not reduced, it could lead to a disease called glaucoma and eventually damage your sight.
2. What you need to know before you use Sturiban Do not use Sturiban:
- if you are allergic to bimatoprost or any of the other ingredients of this medicine (listed in section 6).
- if you have had to stop using eye drops in the past because of a side effect of the preservative benzalkonium chloride.
Warnings and precautions
Talk to your doctor before using Sturiban:
- if you have any breathing problems
- if you have liver or kidney problems
- if you have had a cataract surgery in the past
- if you have dry eye
- if you have or have had any problems with your cornea (front transparent part of the eye)
- if you wear contact lenses (see “Sturiban contains benzalkonium chloride”)
- if you have or have had low blood pressure or low heart rate
- if you have had a viral infection or inflammation of the eye.
Sturiban may cause your eyelashes to darken and grow, and cause the skin around the eyelid to darken too. The colour of your iris may also go darker over time. These changes may be permanent. The change may be more noticeable if you are only treating one eye.
Children and adolescents
Sturiban has not been tested in children under the age of 18 and therefore should not be used by patients under 18 years.
Other medicines and Sturiban
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Sturiban may get into breast milk so you should not breast-feed while you are taking Sturiban.
Driving and using machines
Your sight may become blurred for a short time just after using Sturiban. You should not drive or use machines until your sight is clear again.
Sturiban contains benzalkonium chloride
Do not use the drops when you are wearing your lenses. Wait 15 minutes after using the drops before you put your lenses back in. A preservative in Sturiban called benzalkonium chloride may cause eye irritation and can discolour soft contact lenses.
3. How to use Sturiban
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Sturiban should only be applied to the eye. The recommended dose is one drop of Sturiban in the evening, once daily in each eye that needs treatment.
If you use Sturiban with another eye medicine, wait at least five minutes between using Sturiban and the other eye medicine.
Do not use more than once a day as the effectiveness of treatment may be reduced.
Instructions for use:
You must not use the bottle if the tamper proof seal on the bottle neck is broken before you first use it.
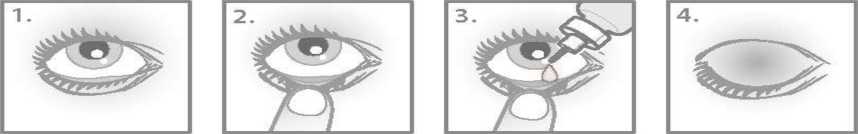
1. Wash your hands. Tilt your head back and look at the ceiling.
2. Gently pull down the lower eyelid until there is a small pocket.
3. Turn the bottle upside down and squeeze it to release one drop into each eye that needs treatment.
4. Let go of the lower lid, and close your eye for 30 seconds.
Wipe off any excess that runs down the cheek. If a drop misses your eye, try again.
To help prevent infections and avoid eye injury, do not let the tip of the bottle touch your eye or anything else. Put the cap back on and close the bottle straight after you have used it.
If you use more Sturiban than you should
If you use more Sturiban than you should, it is unlikely to cause you any serious harm. Put your next dose in at the usual time. If you are worried, talk to your doctor or pharmacist.
If you forget to use Sturiban
If you forget to use Sturiban, use a single drop as soon as you remember, and then go back to your regular routine. Do not take a double dose to make up for a forgotten dose.
If you stop using Sturiban
Sturiban should be used every day to work properly. If you stop using Sturiban the pressure inside your eye may go up, therefore talk to your doctor before stopping this treatment.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common side effects (may affect more than 1 in 10 people):
Affecting the eye
• Slight redness (up to 29% of people).
Common side effects (may affect up to 1 in 10 people):
Affecting the eye
• Small breaks in the surface of the eye (with or without inflammation)
• Irritation
• Itchy eyes
• Longer eyelashes
• Irritation when drop is put in the eye
• Eye pain
Affecting the skin
• Red and itchy eyelids
• Darker skin colour around the eye
• Hair growth around the eye.
Uncommon side effects (may affect up to 1 in 100 people):
Affecting the eye
• Darker Iris colour
• Tired eye
• Swelling of the surface of the eye
• Blurred vision
• Loss of eye lashes
Affecting the skin
• Dry skin
• Crusting on the edge of the eyelid
• Swelling of the eyelid
• Itching
Affecting the body
• Headache
• Feeling of sickness.
Not known (frequency cannot be estimated from the available data):
Affecting the eye
• Macular oedema (swelling of the retina at the back of the eye which may lead to worsening vision)
• Darker eyelid colour
• Eyes appear sunken
• Dryness
Affecting the body
• Asthma
• Worsening of asthma
• Worsening of the lung disease called chronic obstructive pulmonary disease (COPD)
• Shortness of breath
• Symptoms of allergic reaction (swelling, redness of the eye and rash of the skin)
In addition to the side effects for bimatoprost 0.1mg/ml, the following side effects have been seen with another medicine containing a higher strength of bimatoprost (0.3mg/ml):
• Dizziness
• Ocular burning
• An allergic reaction in the eye
• Inflamed eyelids
• Difficulty in seeing clearly
• Worsening of vision
• Swelling of the see-through layer that covers the eye
• A feeling that something is in your eye
• Sensitivity to light
• Tears
• Sticky eyes
• Darker eyelashes
• Retinal bleeding
• Inflammation within the eye
• Cystoid macular oedema (swelling of the retina within the eye leading to worsening vision)
• Eyelid twitching
• Eyelid shrinking, moving away from surface of the eye
• Skin redness around the eye
• Increased blood pressure
• Weakness
• An increase in blood-test results that show how your liver is working.
Other side effects reported with eye drops containing phosphates.
In very rare cases, some patients with severe damage to the clear layer at the front of the eye (the cornea) have developed cloudy patches on the cornea due to calcium build-up during treatment.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme Website: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Sturiban
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bottle label and carton after EXP. The expiry date refers to the last day of that month.
You must throw away the bottle at the latest four weeks after you first opened it, even if there are still some drops left. This will prevent infections.
This medicine does not require any special storage conditions.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Sturiban contains
- The active substance is bimatoprost. One ml of solution contains 0.1mg bimatoprost.
- The other ingredients are benzalkonium chloride (preservative), sodium chloride, sodium phosphate dibasic heptahydrate, citric acid monohydrate and water for injection. Small amounts of sodium hydroxide or hydrochloric acid (concentrated) may be added to keep the level of acid (pH levels) normal.
What Sturiban looks like and contents of the pack
Sturiban is a clear, colourless eye drop solution free from visible particulate matter, in a pack containing 1 or 3 bottles each with a screw cap. The cap has a tamper evident ring. Each bottle contains 3 millilitres of solution.
Marketing Authorisation Holder
Actavis Group PTC ehf.
Reykjavikurvegi 76-78 220 Hafnarfjordur Iceland
Manufacturer
Actavis Group PTC ehf.
Reykjavikurvegi 76-78,
220 Hafnarfjordur Iceland
This leaflet was last revised in August 2016.
If you would like a leaflet with larger text,
Pil Spec no
Logo Actavis
Actavis, Barnstaple, EX32 8NS, UK
6