Subutex 2Mg Sublingual Tablets
5mm 80mm 10mm 75mm 5mm 5mm 75mm 10mm 80mm 5mm
5mm 80mm 10mm 75mm 5mm 5mm 75mm 10mm 80mm 5mm
flOWTO^TORESUBUTEJC
As with all medicines, keep out of the isight and reach of children. iThe tablets should be stored below 30°C in the original package.
,Do not use after the date which is ptamped on the pack.
6. FURTHER INFORMATION -What Subutex contains
The active substance is buprenorphine '(as buprenorphine hydrochloride).
'Each sublingual tablet contains 0.4mg, '2mg or 8mg of buprenorphine.
The other ingredients are monohydrated ilactose, mannitol, maize starch, ipovidone K30, citric acid, magnesium stearate and sodium citrate.
What Subutex looks like and contents of the pack
iSubutex 0.4mg sublingual tablets are luncoated oval white tablets of 8 mm x 4 mm, debossed with "04" on one side. 'Subutex 2mg sublingual tablets are 'uncoated oval white tablets of 10 mm x '5 mm, debossed with "B2" on one side. 'Subutex 8mg sublingual tablets are Xi rrcoated oval white tabtet5T>f14mmx“ 7 mm, debossed with "B8" on one side. The sublingual tablets come in blister 'packs containing either 7 or 28 tablets. Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Indivior UK Limited 103-105 Bath Road
plough---------------
Berkshire SL1 3UH UK
Manufacturer:
Reckitt Benckiser Healthcare (UK)
Limited 'Dansom Lane 'Hull, HU8 7DS
UK_________________
For any information about this medicine, 'please contact the Marketing 'Authorisation Holder:
Telephone +800 270 81 901
'PatientSafetyRoW@indivior.com
this leaflet was last revised in July 2015.
PACKAGE LEAFLET: INFORMATION FORTHE USER Subutex 0.4 mg, 2 mg and 8 mg sublingual tablets
buprenorphine
Read aN of this leaflet carefully before
confusion or hallucinations caused by alcohol.
If you are breastfeeding a baby.
Take special care with Subutex
Tell your doctor before you start taking [Subutex if you have:
[• asthma or other breathing problems H any liver disease such as hepatitis !• low blood pressure
recently suffered hoad4njury-or-bram -disease
a urinary disorder (especially linked to enlarged prostate in men) any kidney disease thyroid problems
adrenocortical disorder (e.g. Addison's disease)
"3D2JJ17Z
Some medicines may increaseThe side-[effects of Subutex and may sometimes pause very serious reactions. Do not ;take any other medicines whilst taking ;Subutex without first talking to your ;doctor, especially:
Benzodiazepines (used to treat anxiety or sleep disorders) such as diazepam, temazepam, alprazolam. Your doctor wiM prescribe the cojrectdose for:you.
Vou start taking this medicine.
K Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
•_ If an\tof the_sida effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Subutex is and what it is used for
2. Before you take Subutex
3. How to take Subutex
4. Possible side sffeets-----
5. How to store Subutex
6. Further information
1. WHAT SUBUTEX IS AND WHAT IT IS USED FOR
Subutex is used to treat dependence on opiate (narcotic) drugs, such as [morphine and heroin in drug addicts Who have agreed to be treated for their
[addiction:---------------
Subutex is used in adults and [adolescents over 16 years of age who [are also receiving medical, social and [psychological support.
j2. BEFORE YOU TAKE SUBUTEX [Do not take Subutex:
If you are a child under the age of 16 years.
If you" are-af1ergic (hypersensrtivefto “ buprenorphine or to any of the other ingredients of this medicine (listed in section 6).
If you have serious breathing problems.
If you have serious problems with your liver.
If you are intoxicated due to alcohol or l^ayejtrernblmcj^weating, anxiety^
Important things to be aware of:
Misuse, abuse and diversion
This medicine can be a target for people who abuse prescription medicines, and should be kept in a safe place to protect it from theft. Do not give this medicine to anyone else. It can cause death_or otherwise_harm_ them.
Breathing problems
Some people have died from respiratoryfailure (inabilityto breathe) because they misused this medicine or took it in combination with other central nervous system depressants, such as alcohol, benzodiazepines (tranquilisers), or other opioids.
Dependence------------
This product can cause dependence. Withdrawal symptoms This product can cause withdrawal symptoms if you take it less than 6 hours after you use a short-acting opioid (e.g. morphine, heroin) or less than 24 hours after you use a long-acting opioid such as methadone.
Subutex«an also ^ause withdrawal- -symptoms if you stop taking it abruptly.
Liver damage
Liver damage has been reported after taking Subutex, especially when the medicine is misused.This could also be due to viral infections (chronic hepatitis C), alcohol abuse, anorexia or use of other medicines with the ability to harm your liver (see section 4). Regular blood tests may be conducted by your doctor to monitor the condition of your liver. Tell your doctor if you have any liver problems before you start treatment with Subutex. Blood pressure
This product may cause your blood pressure to drop suddenly, causing
you toTeel^lizzyJf-yeu-get-upteo---
quickly from sitting or lying down. Diagnosis of unrelated medical conditions
This medicine may mask pain symptoms that could assist in the diagnosis of some diseases. Do not forget to advise your doctor if you take this medicine.
Taking other medicines
Taking the wrong dose of benzodiazepines may cause death duel to respiratory failure (inability to breathe).
Other medicines that may make you feel sleepy which are used to treat illnesses such as anxiety,
________________INQ00339I
sleeplessness, convulsions / seizures, pain.These types of medicines will reduce your alertness levels making it difficult for you to drive and use machines.They may also cause central nervous system depression, which is very serious. Below is a list of _examples of these types of medicines: + - other opioid containing medicines such as methadone, certain pain killers and cough suppressants.
- antidepressants (used to treat depression) such as isocarboxazide, phenelzine, selegiline, tranylcypromine, and valproate may increase the effects of this medicine.
- sedative Hi receptor antagonists
_ Jused lolreataller gic_re_a ct ions)__
such as diphenhydramine and chlorphenamine.
- barbiturates (used to cause sleep or sedation) such as phenobarbital, secobarbital.
- tranquilisers (used to cause sleep or sedation) such as chloral hydrate.
Naltrexone may prevent Subutex from working. If you take naltrexone whilst
+ -you-are taking Subutexyou may---
experience a sudden onset of prolonged and intense withdrawal symptoms.
Clonidine (used to treat high blood pressure) may extend the effects of this medicine. j» Anti-retrovirals (used to treat AIDS) such as ritonavir, nelfinavir, indinavir ± may increase the effects of this “medicine. “
Some antifungal agents (used to treat fungal infections) such as ketoconazole and itraconazole and certain antibiotics (macrolide) may extend the effects of this medicine. Some medicines may decrease the effect of Subutex.These include medicines used to treat epilepsy (such -as-carbamazepine and phenytoin) and medicines used to treat tuberculosis (rifampicin).
To get the greatest benefit from taking Subutex, you must tell your doctor 'about all the medicines you are taking, including alcohol, medicines containing alcohol, street drugs, and any 'prescription medicine you are taking ithat has not been prescribed for you by
nyou+doetor.-------------
Taking Subutex with food and drink 'Alcohol may increase drowsiness and 'may increase the risk of respiratory failure (inability to breathe) if taken with Subutex. Do not take Subutex together iwith alcohol. Do not swallow or consume food or drink until the tablet is completely dissolved.
Pregnancy and breast-feeding_____
Tell your doctor if you are pregnant or lintend to become pregnant.
When taken during pregnancy, 'particularly late pregnancy, medicines 'like Subutex may cause drug withdrawal isymptoms including problems with 'breathing in your new born baby.
^IFTese symptoms may occur several [days after birth.
;Do not breast feed your baby whilst taking this medicine as Subutex passes into breast milk.
|Ask your doctor or pharmacist for advice before taking any medicine.
Driving -and using machines-------
[If you feel drowsy or dizzy while taking [these tablets do not use machinery.
The medicine can affect your ability to [drive as it may make you sleepy or dizzy. Do not drive while taking this medicine until you know how it affects you.
It is an offence to drive if this medicine affects your ability to drive.
However, you wautdmot ber committing an offence if: o The medicine has been prescribed to treat a medical or dental problem and
o You have taken it according to the instructions given by the prescriber or in the information jarovidedwith “tReTriedicine aTicf “ o It was not affecting your ability to drive safely Talk to your doctor or pharmacist if you [are not sure whether it is safe for you to [drive while taking this medicine. [Important information about some of [the ingredients of Subutex [Subutex contains lactose. If you have been told fay-your doeter that -you -have-;an intolerance to some sugars, contact your doctor before taking this medicine.
3. HOWTOTAKE SUBUTEX
l
[You must place the tablet under your [tongue (sublingual) and allow it to [dissolve, which will take 5 to 10 minutes, [This is the only way to take the tablets. Do not chew or swallow them whole, as
hey will rrotrwork.----------
[Your doctor will tell you how many [tablets to take and you should always [follow this advice.
To avoid sudden withdrawal symptoms, [treatment with Subutex should be given [when there are already clear signs of [withdrawal symptoms.
Adults and children over the age of 16 years: when-beginning-treatmentlhe [dose is between 0.8 to 4mg, taken once [a day.
For drug addicts who have not had any Withdrawal treatment: one dose of ISubutex should be taken at least 6 hours [after the last use of the opioid (narcotic [such as morphine or heroin), or when the first signs of craving appear. If you take it less than six hours after you use a narcolic you may gerwlthdrawat “ [symptoms.
[For patients taking methadone: before beginning treatment, your doctor should reduce your dose of methadone to not more than 30mg a day. Subutex may [cause withdrawal symptoms in patients
who are dependent on methadone if jused within 24 hours of the last dose of methadone.
[During your treatment, your doctor may [increase your dose of Subutex, to a [maximum single daily dose of 32mg, depending upon your response. Once |you have_been stable for_a while,your. _ doctor will gradually reduce your dose |and it may be possible to stop it altogether. Do not suddenly stop taking !the tablets, as this may cause Withdrawal symptoms.
|lf you take more Subutex than you should
[If you or someone else takes too much ;of this medicine, you must go or be
|
— |
r — |
— |
— |
takerrimmed'ratelyto an |
|
emergency centre or hospital | ||||
|
as overdose with Subutex may | ||||
|
ra u |
to u |
cause serious and | ||
|
< V |
< V |
life-threatening breathing | ||
|
u lL |
V it |
problems. | ||
|
X £ |
X I* |
If you forget to take Subutex | ||
|
Tell your doctor as soon as | ||||
|
possible if you miss a dose and |
follow his-ox hen instructions- -Do not take a double dose to make up [for the forgotten dose.
[If you stop taking Subutex !Do not change the treatment in any way lor stop treatment without the agreement lof the doctor who is treating you. Stopping treatment suddenly may cause Withdrawal symptoms.
Iltyou-have .any further_questions-on-tbe-l luse of this product, ask your doctor or Ipharmacist.
[How to remove the tablet from the blister pack
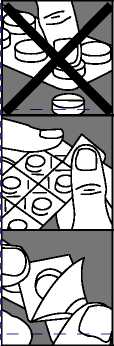
1 - Remove just one section from the blister pack, tearing it along the perforated line.
2 - Starting from the edge where the seal is lifted, pull back the foil on the back to remove the tablet.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Subutex can cause Iside effects, although not everybody Igets them.
Tell your doctor immediately or seek urgent medical attention if you
experience side effects such as:
sudden wheezing, d iff icujty breath ingL swelling of the eyelids, face, tongue, lips, throat or hands; rash or itching especially those covering your whole body.These may be signs of a life-threatening allergic reaction, if you start to breathe more slowly or weakly than expected (respiratory depression!
if you start to Teel Taint, as this may lae a sign of low blood pressure.
Also tell your doctor immediately if you [experience side effects such as:
• severe fatigue (tiredness), have no appetite or if your skin or eyes look yellow.These may be symptoms of
live rda mage,____________
The frequency of possible side effects listed below is defined using the following convention: very common (affects more than 1 user in 10)
common (affects 1 to 10 users in 100) not known (frequency cannot be estimated from the available data).
Side effects reported with Subutex
Very common side effects:
Drug withdrawal syndrome, headache, hyperhidrosis (sweating), insomnia (inability to sleep), nausea (feeling sick), pain_
Common side effects:
Abdominal pain, agitation, anxiety, joint pain, weakness, back pain, bone pain, bronchitis, chest pain, chills,
constipation,-cough; decreased----
appetite, depression, diarrhoea, dizziness, dry mouth, painful period, indigestion, shortness of breath, flatulence, gastrointestinal disorder, hostility, increase in muscle tension, infection, influenza, nervousness, tearing (watery eyes) disorder, swollen glands (lymph nodes), malaise, migraine, muscje spasrns^rnusc|e pahv dilation of the pupil, neck pain, palpitations, paranoia, burning or tingling in hands and feet, swelling (hands and feet), sore throat and painful swallowing, fever, rash, somnolence, syncope (fainting), thinking abnormal, tooth disorder, tremor; flushing, vomiting (being sick), yawning.
~Frequency~not know nr
Drug dependence, drug withdrawal syndrome in newborn, hallucinations (sensing things that are not real), drop in blood pressure on changing position from sitting or lying down to standing, difficulty in urinating.
Misusing this medicine by injecting it can cause withdrawal symptoms,
infections,.other skin reactions_and___
potentially serious liver problems.
f any of the side effects gets serious, or [if you notice any side effects not listed in [this leaflet, please tell your doctor or [pharmacist.
[Reporting of side effects
[If you get any side effects, talk to your [doctor, pharmacist or nurse.This [includes any possible side effects not listed in this leaflet. You can also report-[side effects directly via theYellow Card [Scheme at:
www.mhra.gov.uk/yellowcard [By reporting side effects you can help [provide more information on the safety [of this medicine.
CUSTOMER INFO: Minimum Point Size = 10.00pt
lndivior Artwork
|
and Print Specification | |
|
Trident Reference No: TRIND191325 | |
|
ZEN Ref: |
TR983123 |
|
Action: |
A |
|
Brand: |
Buprenorphine |
|
Category: |
Treatment |
|
Segment Group: |
Subutex |
|
Segment: |
0.4mg, 2mg & 8mg |
|
Pack Size: |
N/A- |
|
Market/Country: |
UK |
|
Date: |
29/06/15 |
|
RBH Contact: |
John Mouatt IND |
|
Artwork Type: Commercial | |
|
Component Code (2D if applicable): IND00339 | |
|
Parent Technical Packaging | |
|
Specification: |
D0081679 |
|
Supplier Code: |
3020172 |
|
Supply Point: |
RB |
|
Pharmacode No/NE: |
010010(81) |
|
Edgemark Position: |
N/A |
|
CAD Cam Ref: |
Sub~Lflt-D0081 G79-340x298mm |
|
Printer: |
Essentra Packaging (Bradford, UK) |
|
Substrate: |
Paper White |
|
Technical Et Non Printing Items | |
|
|_| Cutter |
| | Cutter 2 (if applicable) |
|
HI Guides |
| | Guides 2 (if applicable) |
|
Colours | |
BARCODE 11MF0
Barcode Type: N/A
Barcode Number: N/A
Magnification: N/A
Truncated By: N/A
Full Height: N/A
Bar Height (Smallest Bar): N/A
BWR: N/A
Encoded Data: N/A
INDIVIOR
Focus on you.
TRIDENT
Connaught House, Connaught Road, Kingswood Business Park, Hull, HU7 3AP, England. T: +44(0) 1482 828100
Please note that any low resolution paper Canon colour copies associated with this job should be reTerred to for content, layout and colour separation only.
STUDIO USE ONLY Samantha Keyworth vi.o
Sm«Art check results: G=1; 0=0; R=0; -SK- 29/06/15 12:12:22
ARTWORK APPROVAL
Approved by Sonoco Trident on behalf of lndivior
Signed:__________________
Date:___________________
NOT APPROVED FOR COLOUR
UNDER NO CIRCUMSTANCES SHOULD THIS ARTWORK BE ALTERED WITHOUT PRIOR PERMISSION FROM TRIDENT.
|
Production line | |||
|
Manufacturing site | |||
|
Pack Tech approver |
1 st 2nd | ||
|
Date | |||
|
Check Criteria |
Details Ft Comments |
Checks | |
|
Approvals |
1st |
2nd | |
|
Market Approval | |||
|
Regulatory Approval | |||
|
3rd Party Approval | |||
|
Technical Drawing/Cutter ref no. | |||
|
PPI component code correct on artwork? | |||
|
Correct drawing for the production line? | |||
|
Profile shape Et dimensions | |||
|
Graphics layout/orientation | |||
|
Unwind diagram (PPTD1022/1 -20) | |||
|
Printable areas/bleed | |||
|
Varnish free areas | |||
|
Variable coding position Ft dimensions | |||
|
Varnish and print requirements | |||
|
Pre-printed headers (alignment and suitability) | |||
|
Embossing, Braille Et Foil Blocking | |||
|
Separations on artwork | |||
|
Alignment within cutter | |||
|
Braille structure | |||
|
Verification Code Type; Pharma Code / 2D / OCV | |||
|
Bar sequence and/or number correct Position | |||
|
Size (height, width, spacing, font) | |||
|
Direction of read | |||
|
Light margins and print free areas | |||
|
Colour | |||
|
Repeat distance (reeled material) | |||
|
Cross check related docs ie BOM | |||
|
Bar code Type; EAN, ITF, Code39, etc | |||
|
Magnification / width and height | |||
|
Colour (e.g. black print on white background) | |||
|
Light margins Ft indicators | |||
|
Bearer bars Et H gauges (if required) | |||
|
Symbol grade (Pass = A,B,C; Action = D,F) | |||
|
Bar code number (human readable) | |||
|
Bar code number (encoded Ft hidden data) | |||
|
Confirm bar code number from source | |||
|
Cross check related docs (JDE/PID) | |||
|
Photo-electric cell mark / edge marks | |||
|
Present Ft per Tech drawing? | |||
|
Correct pitch? | |||
|
Additional markings | |||
|
| e-mark, recycle logo etc | | |||
|
Legend box (RB specification) | |||
|
Correct component code | |||
|
Correct D spec | |||
|
Correct printer | |||
|
Correct Substrate | |||
|
A/W is set up appropriately for this substrate | |||
|
Edge mark details | |||
|
Pharmacode details | |||
|
Colours Et print process | |||
|
Varnish | |||
|
Barcode information | |||
|
General details are correct | |||
|
Pass / Fail comments and Signature | |||
|
By approving the artwork referenced within this form 1 authorise it for release to the print supplier | |||
|
Additional Comments | |||
Barcode Type: Barcode Number: Magnification: Truncated By:
Full Height:
Bar Height (Smallest Bar): N/A
BWR: N/A
Encoded Data: N/A
TRIDENT
Connaught House, Connaught Road, Kingswood Business Park, Hull, HU7 3AP, England. T: +44(0) 1482 828100
Please note that any low resolution paper Canon colour copies associated with this job should be referred to for content, layout and colour separation only.
STUDIO USE ONLY Samantha Keyworth vi.o
Sm«Art check results: G=1; 0=0; R=0; -SK- 29/06/15 12:12:22
Approved by Sonoco Trident on behalf of lndivior
Signed:__________________
Date:___________________
Version 002 20/2/2015
|
I Trident Reference No: TRIND191325 I | |
|
ZEN Ref: |
TR983123 |
|
Action: |
A |
|
Brand: |
Buprenorphine |
|
Category: |
Treatment |
|
Segment Group: |
Subutex |
|
Segment: |
0.4mg, 2mg & 8mg |
|
Pack Size: |
N/A- |
|
Market/Country: |
UK |
|
Date: |
29/06/15 |
|
RBH Contact: |
John Mouatt IND |
|
1 Artwork Type: G | |
|
1 Component Code (2D if applicable): IND00339 | |
|
1 Parent Technical Packaging | |
|
Specification: |
D0081679 |
|
Supplier Code: |
3020172 |
|
Supply Point: |
RB |
|
Pharmacode No/NE: |
010010(81) |
|
Edgemark Position: |
N/A |
|
CAD Cam Ref: |
Sub-Lflt-D0081 G79-340x298mm |
|
Printer: |
Essentra Packaging (Bradford, UK) |
|
Substrate: |
Paper White |
|
1 Technical Et Non Printing Items 1 | |
|
1 Cutter |
| | Cutter 2 (if applicable) 1 |
|
| | Guides |
| | Guides 2 (if applicable) 1 |
Colours
N/A
N/A
N/A
N/A
N/A
NOT APPROVED FOR COLOUR
UNDER NO CIRCUMSTANCES SHOULD THIS ARTWORK BE ALTERED WITHOUT PRIOR PERMISSION FROM TRIDENT.
O
' CT> CN
O ■ 00
CN
O
■
CN
o
- UO CN
O - LO
CN
O - N-
CN
O
- on CN
O
- CN CN
O
CN
O
-o
CN
o
- CO
O - LO
o
- N-
o
- CN
O
-o
_o
O
. O 00
. o
o
<D
O
LO
o
O
co
o
CN
-=_O