Subutex 8Mg Sublingual Tablets
S603 LEAFLET Subutex 20160816 Taking other medicines
PACKAGE LEAFLET: INFORMATION FOR THE USER
SUBUTEX® 8mg SUBLINGUAL TABLETS (buprenorphine hydrochloride)
Your medicine is known as Subutex 8mg sublingual tablets but will be referred to as Subutex throughout the following leaflet. Information for other strength of Subutex is also available in this leaflet.
Read all of this leaflet carefully before you start taking this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Subutex is and what it is used for
2. Before you take Subutex
3. How to take Subutex
4. Possible side effects
5. How to store Subutex
6. Further information
1. WHAT SUBUTEX IS AND WHAT IT IS USED FOR
Subutex is used to treat dependence on opiate (narcotic) drugs, such as morphine and heroin in drug addicts who have agreed to be treated for their addiction.
Subutex is used in adults and adolescents over 16 years of age who are also receiving medical, social and psychological support.
2. BEFORE YOU TAKE SUBUTEX
Do not take Subutex:
• If you are a child under the age of 16 years.
• If you are allergic (hypersensitive) to buprenorphine or to any of the other ingredients of this medicine (listed in section 6).
• If you have serious breathing problems.
• If you have serious problems with your liver.
• If you are intoxicated due to alcohol or have trembling, sweating, anxiety confusion or hallucinations caused by alcohol.
• If you are breast-feeding a baby.
Take special care with Subutex
Tell your doctor before you start taking Subutex if you have:
• asthma or other breathing problems
• any liver disease such as hepatitis
• low blood pressure
• recently suffered head injury or brain disease
• a urinary disorder (especially linked to enlarged prostate in men)
• any kidney disease
• thyroid problems
• adrenocortical disorder (e.g. Addison's disease)
Important things to be aware of:
• Misuse, abuse and diversion
This medicine can be a target for people who abuse prescription medicines, and should be kept in a safe place to protect it from theft. Do not give this medicine to anyone else. It can cause death or otherwise harm them.
• Breathing problems
Some people have died from respiratory failure (inability to breathe) because they misused this medicine or took it in combination with other central nervous system depressants, such as alcohol, benzodiazepines (tranquilisers), or other opioids.
• Dependence
This product can cause dependence.
• Withdrawal symptoms
This product can cause withdrawal symptoms if you take it less than 6 hours after you use a short-acting opioid (e.g. morphine, heroin) or less than 24 hours after you use a long-acting opioid such as methadone.
Subutex can also cause withdrawal symptoms if you stop taking it abruptly.
• Liver damage
Liver damage has been reported after taking Subutex, especially when the medicine is misused. This could also be due to viral infections (chronic hepatitis C), alcohol abuse, anorexia or use of other medicines with the ability to harm your liver (see section 4). Regular blood tests may be conducted by your doctor to monitor the condition of your liver. Tell your doctor if you have any liver problems before you start treatment with Subutex.
• Blood pressure
This product may cause your blood pressure to drop suddenly, causing you to feel dizzy if you get up too quickly from sitting or lying down.
• Diagnosis of unrelated medical conditions
This medicine may mask pain symptoms that could assist in the diagnosis of some diseases. Do not forget to advise your doctor if you take this medicine.
Some medicines may increase the side effects of Subutex and may sometimes cause very serious reactions. Do not take any other medicines whilst taking Subutex without first talking to your doctor, especially:
• Benzodiazepines (used to treat anxiety or sleep disorders) such as diazepam, temazepam, alprazolam. Your doctor will prescribe the correct dose for you. Taking the wrong dose of benzodiazepines may cause death due to respiratory failure (inability to breathe).
• Other medicines that may make you feel sleepy which are used to treat illnesses such as anxiety, sleeplessness, convulsions/seizures, pain. These types of medicines will reduce your alertness levels making it difficult for you to drive and use machines. They may also cause central nervous system depression, which is very serious. Below is a list of examples of these types of medicines:
• other opioid containing medicines such as methadone, certain pain killers and cough suppressants.
• antidepressants (used to treat depression) such as isocarboxazide, phenelzine, selegeline, tranylcypromine, and valproate may increase the effects of this medicine.
• sedative H receptor antagonists (used to treat allergic reactions) such as diphenhydramine and chlorphenamine.
• barbiturates (used to cause sleep or sedation) such as phenobarbital, secobarbital.
• tranquilisers (used to cause sleep or sedation) such as chloral hydrate.
• Naltrexone may prevent Subutex from working. If you take naltrexone whilst you are taking Subutex you may experience a sudden onset of prolonged and intense withdrawal symptoms.
• Clonidine (used to treat high blood pressure) may extend the effects of this medicine.
• Anti-retrovirals (used to treat AIDS) such as ritonavir, nelfinavir, indinavir may increase the effects of this medicine.
• Some antifungal agents (used to treat fungal infections) such as ketoconazole and itraconazole and certain antibiotics (macrolide) may extend the effects of this medicine.
• Some medicines may decrease the effect of Subutex. These include medicines used to treat epilepsy (such as carbamazepine and phenytoin) and medicines used to treat tuberculosis (rifampicin).
To get the greatest benefit from taking Subutex, you must tell your doctor about all the medicines you are taking, including alcohol, medicines containing alcohol, street drugs, and any prescription medicine you are taking that has not been prescribed for you by your doctor.
Taking Subutex with food and drink
Alcohol may increase drowsiness and may increase the risk of respiratory failure (inability to breathe) if taken with Subutex. Do not take Subutex together with alcohol. Do not swallow or consume food or drink until the tablet is completely dissolved.
Pregnancy and breast-feeding
Tell your doctor if you are pregnant or intend to become pregnant.
When taken during pregnancy, particularly late pregnancy, medicines like Subutex may cause drug withdrawal symptoms including problems with breathing in your new born baby. These symptoms may occur several days after birth.
Do not breast-feed your baby whilst taking this medicine as Subutex passes into breast milk.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
If you feel drowsy or dizzy while taking these tablets do not use machinery.
The medicine can affect your ability to drive as it may make you sleepy or dizzy.
• Do not drive while taking this medicine until you know how it affects you.
• It is an offence to drive if this medicine affects your ability to drive.
• However, you would not be committing an offence if:
o The medicine has been prescribed to treat a medical or dental problem and
o You have taken it according to the instructions given by the prescriber or in the information provided with the medicine and
o It was not affecting your ability to drive safely
Talk to your doctor or pharmacist if you are not sure whether it is safe for you to drive while taking this medicine.
Important information about some of the ingredients of Subutex
Subutex contains lactose. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine.
3. HOW TO TAKE SUBUTEX
You must place the tablet under your tongue (sublingual) and allow it to dissolve, which will take 5 to 10 minutes. This is the only way to take the tablets. Do not chew or swallow them whole, as they will not work.
Your doctor will tell you how many tablets to take and you should always follow this advice.
To avoid sudden withdrawal symptoms, treatment with Subutex should be given when there are already clear signs of withdrawal symptoms.
Adults and children over the age of 16 years: when beginning treatment the dose is between 0.8 to 4mg, taken once a day.
For drug addicts who have not had any withdrawal treatment:
one dose of Subutex should be taken at least 6 hours after the last use of the opioid (narcotic such as morphine or heroin), or when the first signs of craving appear. If you take it less than six hours after you use a narcotic you may get withdrawal symptoms.
For patients taking methadone: before beginning treatment, your doctor should reduce your dose of methadone to not more than 30mg a day. Subutex may cause withdrawal symptoms in patients

who are dependent on methadone if used within 24 hours of the last dose of methadone.
During your treatment, your doctor may increase your dose of Subutex, to a maximum single daily dose of 32mg, depending upon your response. Once you have been stable for a while, your doctor will gradually reduce your dose and it may be possible to stop it altogether. Do not suddenly stop taking the tablets, as this may cause withdrawal symptoms.
If you take more Subutex than you should If you or someone else takes too much of this medicine, you must go or be taken immediately to an emergency centre or hospital as overdose with Subutex may cause serious and life-threatening breathing problems.
If you forget to take Subutex
Tell your doctor as soon as possible if you miss a dose and follow his or her instructions. Do not take a double dose to make up for the forgotten dose.
If you stop taking Subutex
Do not change the treatment in any way or stop treatment without the agreement of the doctor who is treating you. Stopping treatment suddenly may cause withdrawal symptoms.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
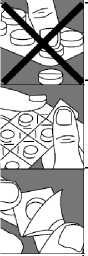
How to remove the tablet from the blister pack
|
^ \ |
1- Remove just one section from the blister pack, tearing it along the perforated line. |
|
2 - Starting from the edge where the seal is lifted, pull back the foil on the back to remove the tablet. |
4. POSSIBLE SIDE EFFECTS
Like all medicines, Subutex can cause side effects, although not everybody gets them.
Tell your doctor immediately or seek urgent medical attention if
you experience side effects such as:
• sudden wheezing, difficulty breathing, swelling of the eyelids, face, tongue, lips, throat or hands; rash or itching especially those covering your whole body. These may be signs of a life-threatening allergic reaction.
• if you start to breathe more slowly or weakly than expected (respiratory depression).
• if you start to feel faint, as this may be a sign of low blood pressure.
Also tell your doctor immediately if you experience side effects such as:
• severe fatigue (tiredness), have no appetite or if your skin or eyes look yellow. These may be symptoms of liver damage.
The frequency of possible side effects listed below is defined using the following convention:
• very common (affects more than 1 user in 10)
• common (affects 1 to 10 users in 100)
• not known (frequency cannot be estimated from the available
data)._
Side effects reported with Subutex_
Very common side effects:_
Drug withdrawal syndrome, headache, hyperhidrosis (sweating), insomnia (inability to sleep), nausea (feeling sick), pain_
Common side effects:_
Abdominal pain, agitation, anxiety, joint pain, weakness, back pain, bone pain, bronchitis, chest pain, chills, constipation, cough, decreased appetite, depression, diarrhoea, dizziness, dry mouth, painful period, indigestion, shortness of breath, flatulence, gastrointestinal disorder, hostility, increase in muscle tension, infection, influenza, nervousness, tearing (watery eyes) disorder, swollen glands (lymph nodes), malaise, migraine, muscle spasms, muscle pain, dilation of the pupil, neck pain, palpitations, paranoia, burning or tingling in hands and feet, swelling (hands and feet), sore throat and painful swallowing, fever, rash, somnolence, syncope (fainting), thinking abnormal, tooth disorder, tremor; flushing, vomiting (being sick), yawning_
Frequency not known:_
Drug dependence, drug withdrawal syndrome in newborn, hallucinations (sensing things that are not real), drop in blood pressure on changing position from sitting or lying down to standing, difficulty in urinating.
Misusing this medicine by injecting it can cause withdrawal symptoms, infections, other skin reactions and potentially serious liver problems._
5. HOW TO STORE SUBUTEX
• KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
• Do not store above 30°C. Stored in the original package to protect from moisture.
• Do not use after the expiry date printed on the carton label or blister strip.
• If your doctor tells you to stop using the medicine, please take it back to the pharmacist for safe disposal. Only keep the medicine if your doctor tells you to.
• If the medicine becomes discoloured or shows any other signs of deterioration, you should seek the advice of your pharmacist who will tell you what to do.
6. FURTHER INFORMATION
What Subutex contains
• Each Subutex 8mg Sublingual Tablet contains the active ingredient buprenorphine hydrochloride equivalent to 8mg buprenorphine base.
• Subutex Tablets also contain the following inactive ingredients: lactose monohydrate, mannitol, maize starch, povidone K30, citric acid, sodium citrate and magnesium stearate.
What Subutex looks like and contents of the pack
Subutex 8mg Sublingual Tablets are oval white tablets are marked
‘B8' on one side with a sword logo on the other.
Subutex Tablets are available as blister packs of 7 tablets
Product Licence holder
Procured from within the EU and repackaged by the Parallel Import
Product Licence holder: S&M Medical Ltd, Chemilines House,
Alperton Lane, Wembley, HA0 1DX.
Manufacturer
This product is manufactured by Reckitt Benckiser Healthcare (UK)
Ltd., Dansom Lane, Hull, HU8 7DS, United Kingdom.
| POM | PL. 19488/0603 Subutex 8mg Sublingual Tablets
Leaflet revision date: 16 August 2016
Subutex® is a registered trade mark of Indivior UK Limited.
S603 LEAFLET Subutex 20160816
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist. Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
PACKAGE LEAFLET: INFORMATION FOR THE USER
BUPRENORPHINE 8mg SUBLINGUAL TABLETS (buprenorphine hydrochloride)
Your medicine is known as Buprenorphine 8mg sublingual tablets but will be referred to as Buprenorphine Sublingual Tablets throughout the following leaflet.
Information for other strength of Buprenorphine Sublingual Tablets is also available in this leaflet.
Read all of this leaflet carefully before you start taking this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Buprenorphine Sublingual Tablets are and what they are used for
2. Before you take Buprenorphine Sublingual Tablets
3. How to take Buprenorphine Sublingual Tablets
4. Possible side effects
5. How to store Buprenorphine Sublingual Tablets
6. Further information
1. WHAT BUPRENORPHINE SUBLINGUAL TABLETS ARE AND WHAT THEY ARE USED FOR
Buprenorphine Sublingual Tablets are used to treat dependence on opiate (narcotic) drugs, such as morphine and heroin in drug addicts who have agreed to be treated for their addiction.
Buprenorphine Sublingual Tablets are used in adults and adolescents over 16 years of age who are also receiving medical, social and psychological support.
2. BEFORE YOU TAKE BUPRENORPHINE SUBLINGUAL TABLETS
Do not take Buprenorphine Sublingual Tablets:
• If you are a child under the age of 16 years.
• If you are allergic (hypersensitive) to buprenorphine or to any of the other ingredients of this medicine (listed in section 6).
• If you have serious breathing problems.
• If you have serious problems with your liver.
• If you are intoxicated due to alcohol or have trembling, sweating, anxiety confusion or hallucinations caused by alcohol.
• If you are breast-feeding a baby.
Take special care with Buprenorphine Sublingual Tablets
Tell your doctor before you start taking Buprenorphine Sublingual Tablets if you have:
• asthma or other breathing problems
• any liver disease such as hepatitis
• low blood pressure
• recently suffered head injury or brain disease
• a urinary disorder (especially linked to enlarged prostate in men)
• any kidney disease
• thyroid problems
• adrenocortical disorder (e.g. Addison's disease)
Important things to be aware of:
• Misuse, abuse and diversion
This medicine can be a target for people who abuse prescription medicines, and should be kept in a safe place to protect it from theft. Do not give this medicine to anyone else. It can cause death or otherwise harm them.
• Breathing problems
Some people have died from respiratory failure (inability to breathe) because they misused this medicine or took it in combination with other central nervous system depressants, such as alcohol, benzodiazepines (tranquilisers), or other opioids.
• Dependence
This product can cause dependence.
• Withdrawal symptoms
This product can cause withdrawal symptoms if you take it less than 6 hours after you use a short-acting opioid (e.g. morphine, heroin) or less than 24 hours after you use a long-acting opioid such as methadone.
Buprenorphine Sublingual Tablets can also cause withdrawal symptoms if you stop taking it abruptly.
• Liver damage
Liver damage has been reported after taking Buprenorphine Sublingual Tablets, especially when the medicine is misused. This could also be due to viral infections (chronic hepatitis C), alcohol abuse, anorexia or use of other medicines with the ability to harm your liver (see section 4). Regular blood tests may be conducted by your doctor to monitor the condition of your liver. Tell your doctor if you have any liver problems before you start treatment with Buprenorphine Sublingual Tablets.
• Blood pressure
This product may cause your blood pressure to drop suddenly, causing you to feel dizzy if you get up too quickly from sitting or lying down.
• Diagnosis of unrelated medical conditions
This medicine may mask pain symptoms that could assist in the diagnosis of some diseases. Do not forget to advise your doctor if you take this medicine.
Taking other medicines
Some medicines may increase the side effects of Buprenorphine Sublingual Tablets and may sometimes cause very serious reactions. Do not take any other medicines whilst taking Buprenorphine Sublingual Tablets without first talking to your doctor, especially:
• Benzodiazepines (used to treat anxiety or sleep disorders) such as diazepam, temazepam, alprazolam. Your doctor will prescribe the correct dose for you. Taking the wrong dose of benzodiazepines may cause death due to respiratory failure (inability to breathe).
• Other medicines that may make you feel sleepy which are used to treat illnesses such as anxiety, sleeplessness, convulsions/seizures, pain. These types of medicines will reduce your alertness levels making it difficult for you to drive and use machines. They may also cause central nervous system depression, which is very serious. Below is a list of examples of these types of medicines:
• other opioid containing medicines such as methadone, certain pain killers and cough suppressants.
• antidepressants (used to treat depression) such as isocarboxazide, phenelzine, selegeline, tranylcypromine, and valproate may increase the effects of this medicine.
• sedative H receptor antagonists (used to treat allergic reactions) such as diphenhydramine and chlorphenamine.
• barbiturates (used to cause sleep or sedation) such as phenobarbital, secobarbital.
• tranquilisers (used to cause sleep or sedation) such as chloral hydrate.
• Naltrexone may prevent Buprenorphine Sublingual Tablets from working. If you take naltrexone whilst you are taking Buprenorphine Sublingual Tablets you may experience a sudden onset of prolonged and intense withdrawal symptoms.
• Clonidine (used to treat high blood pressure) may extend the effects of this medicine.
• Anti-retrovirals (used to treat AIDS) such as ritonavir, nelfinavir, indinavir may increase the effects of this medicine.
• Some antifungal agents (used to treat fungal infections) such as ketoconazole and itraconazole and certain antibiotics (macrolide) may extend the effects of this medicine.
• Some medicines may decrease the effect of Buprenorphine Sublingual Tablets. These include medicines used to treat epilepsy (such as carbamazepine and phenytoin) and medicines used to treat tuberculosis (rifampicin).
To get the greatest benefit from taking Buprenorphine Sublingual Tablets, you must tell your doctor about all the medicines you are taking, including alcohol, medicines containing alcohol, street drugs, and any prescription medicine you are taking that has not been prescribed for you by your doctor.
Taking Buprenorphine Sublingual Tablets with food and drink
Alcohol may increase drowsiness and may increase the risk of respiratory failure (inability to breathe) if taken with Buprenorphine Sublingual Tablets. Do not take Buprenorphine Sublingual Tablets together with alcohol. Do not swallow or consume food or drink until the tablet is completely dissolved.
Pregnancy and breast-feeding
Tell your doctor if you are pregnant or intend to become pregnant.
When taken during pregnancy, particularly late pregnancy, medicines like Buprenorphine Sublingual Tablets may cause drug withdrawal symptoms including problems with breathing in your new born baby. These symptoms may occur several days after birth.
Do not breast-feed your baby whilst taking this medicine as Buprenorphine Sublingual Tablet passes into breast milk.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
If you feel drowsy or dizzy while taking these tablets do not use machinery.
The medicine can affect your ability to drive as it may make you sleepy or dizzy.
• Do not drive while taking this medicine until you know how it affects you.
• It is an offence to drive if this medicine affects your ability to drive.
• However, you would not be committing an offence if:
o The medicine has been prescribed to treat a medical or dental problem and
o You have taken it according to the instructions given by the prescriber or in the information provided with the medicine and
o It was not affecting your ability to drive safely
Talk to your doctor or pharmacist if you are not sure whether it is safe for you to drive while taking this medicine.
Important information about some of the ingredients of Buprenorphine Sublingual Tablets
Buprenorphine Sublingual Tablets contain lactose. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine.
3. HOW TO TAKE BUPRENORPHINE SUBLINGUAL TABLETS
You must place the tablet under your tongue (sublingual) and allow it to dissolve, which will take 5 to 10 minutes. This is the only way to take the tablets. Do not chew or swallow them whole, as they will not work.
Your doctor will tell you how many tablets to take and you should always follow this advice.
To avoid sudden withdrawal symptoms, treatment with Buprenorphine Sublingual Tablets should be given when there are already clear signs of withdrawal symptoms.

Adults and children over the age of 16 years: when beginning treatment the dose is between 0.8 to 4mg, taken once a day.
For drug addicts who have not had any withdrawal treatment:
one dose of Buprenorphine Sublingual Tablets should be taken at least 6 hours after the last use of the opioid (narcotic such as morphine or heroin), or when the first signs of craving appear. If you take it less than six hours after you use a narcotic you may get withdrawal symptoms.
For patients taking methadone: before beginning treatment, your doctor should reduce your dose of methadone to not more than 30mg a day. Buprenorphine Sublingual Tablets may cause withdrawal symptoms in patients who are dependent on methadone if used within 24 hours of the last dose of methadone.
During your treatment, your doctor may increase your dose of Buprenorphine Sublingual Tablets, to a maximum single daily dose of 32mg, depending upon your response. Once you have been stable for a while, your doctor will gradually reduce your dose and it may be possible to stop it altogether. Do not suddenly stop taking the tablets, as this may cause withdrawal symptoms.
If you take more Buprenorphine Sublingual Tablets than you should
If you or someone else takes too much of this medicine, you must go or be taken immediately to an emergency centre or hospital as overdose with Buprenorphine Sublingual Tablets may cause serious and life-threatening breathing problems.
If you forget to take Buprenorphine Sublingual Tablets
Tell your doctor as soon as possible if you miss a dose and follow his or her instructions. Do not take a double dose to make up for the forgotten dose.
If you stop taking Buprenorphine Sublingual Tablets
Do not change the treatment in any way or stop treatment without the agreement of the doctor who is treating you. Stopping treatment suddenly may cause withdrawal symptoms.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
How to remove the tablet from the blister pack 1- Remove just one section from the blister pack, tearing it along the perforated line.
2 - Starting from the edge where the seal is lifted, pull back the foil on the back to remove the tablet.
5. HOW TO STORE BUPRENORPHINE SUBLINGUAL TABLETS
• KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
• Do not store above 30°C. Store in the original package to protect from moisture.
• Do not use after the expiry date printed on the carton label or blister strip.
• If your doctor tells you to stop using the medicine, please take it back to the pharmacist for safe disposal. Only keep the medicine if your doctor tells you to.
• If the medicine becomes discoloured or shows any other signs of deterioration, you should seek the advice of your pharmacist who will tell you what to do.
6. FURTHER INFORMATION
What Buprenorphine Sublingual Tablets contain
• Each Buprenorphine 8mg Sublingual Tablet contains the active ingredient buprenorphine hydrochloride equivalent to 8mg buprenorphine base.
• Buprenorphine Tablets also contain the following inactive ingredients: lactose monohydrate, mannitol, maize starch, povidone K30, citric acid, sodium citrate and magnesium stearate.
What Buprenorphine Sublingual Tablets look like and contents of the pack
Buprenorphine 8mg Sublingual Tablets are oval white tablets are marked ‘B8' on one side with a sword logo on the other.
Buprenorphine Tablets are available as blister packs of 7 tablets Product Licence holder
Procured from within the EU and repackaged by the Parallel Import Product Licence holder: S&M Medical Ltd, Chemilines House, Alperton Lane, Wembley, HA0 1DX.
Manufacturer
This product is manufactured by Reckitt Benckiser Healthcare (UK) Ltd., Dansom Lane, Hull, HU8 7DS, United Kingdom.
| POM | PL. 19488/0603 Buprenorphine 8mg Sublingual Tablets
Leaflet revision date: 16 August 2016
S603 LEAFLET Buprenorphine 20160816
4. POSSIBLE SIDE EFFECTS
Like all medicines, Buprenorphine Sublingual Tablets can cause side effects, although not everybody gets them.
Tell your doctor immediately or seek urgent medical attention if
you experience side effects such as:
• sudden wheezing, difficulty breathing, swelling of the eyelids, face, tongue, lips, throat or hands; rash or itching especially those covering your whole body. These may be signs of a life-threatening allergic reaction.
• if you start to breathe more slowly or weakly than expected (respiratory depression).
• if you start to feel faint, as this may be a sign of low blood pressure.
Also tell your doctor immediately if you experience side effects such as:
• severe fatigue (tiredness), have no appetite or if your skin or eyes look yellow. These may be symptoms of liver damage.
The frequency of possible side effects listed below is defined using the following convention:
• very common (affects more than 1 user in 10)
• common (affects 1 to 10 users in 100)
• not known (frequency cannot be estimated from the available
data)._
Side effects reported with Buprenorphine Sublingual Tablets
Very common side effects:_
Drug withdrawal syndrome, headache, hyperhidrosis (sweating), insomnia (inability to sleep), nausea (feeling sick), pain_
Common side effects:_
Abdominal pain, agitation, anxiety, joint pain, weakness, back pain, bone pain, bronchitis, chest pain, chills, constipation, cough, decreased appetite, depression, diarrhoea, dizziness, dry mouth, painful period, indigestion, shortness of breath, flatulence, gastrointestinal disorder, hostility, increase in muscle tension, infection, influenza, nervousness, tearing (watery eyes) disorder, swollen glands (lymph nodes), malaise, migraine, muscle spasms, muscle pain, dilation of the pupil, neck pain, palpitations, paranoia, burning or tingling in hands and feet, swelling (hands and feet), sore throat and painful swallowing, fever, rash, somnolence, syncope (fainting), thinking abnormal, tooth disorder, tremor; flushing, vomiting (being sick), yawning_
Frequency not known:_
Drug dependence, drug withdrawal syndrome in newborn, hallucinations (sensing things that are not real), drop in blood pressure on changing position from sitting or lying down to standing, difficulty in urinating.
Misusing this medicine by injecting it can cause withdrawal symptoms, infections, other skin reactions and potentially serious liver problems._
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist. Reporting of side effects
If you get any side effects talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
By reporting side effects, you can help provide more information on the safety of this medicine.