Sumatriptan 6 Mg/0.5 Ml Solution For Injection
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
Package Leaflet: Information for the patient
Sumatriptan Injection is usually injected into the thigh.
Read all of this leaflet carefully before you start using this medicine medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Sumatriptan 6 mg/0.5 ml Solution for Injection
Sumatriptan
1. What Sumatriptan Injection is and what it is used for
2. What you need to know before you use Sumatriptan Injection
3. How to use Sumatriptan Injection
4. Possible side effects
5. How to store Sumatriptan Injection
6. Contents of the pack and other information
1. What Sumatriptan Injection is and what it is used for
The active substance in Sumatriptan Injection is Sumatriptan. It is one of a group of medicines called 5HT, receptor agonists.
Sumatriptan Injection is used to treat migraine headache and a rare condition called cluster headache. The symptoms of migraine may due to temporary swelling of blood vessels in the head. Sumatriptan is believed to work by reducing the size of these blood vessels.
2. What you need to know before you use Sumatriptan Injection Do not use Sumatriptan Injection
- If you are allergic to Sumatriptan or any of the other ingredients of this medicine (listed in Section 6).
- If you have heart problems or you already had a heart attack
- If you have circulation problems in your arms and legs
- If you have had a stroke or a mini-stroke (also called a transient ischaemic attack or TIA)
- If you have serious liver disease
- If you have severe or uncontrolled high blood pressure.
- With other migraine medicines containing ergotamine, or similar medicines like methylsergide, or any triptan or 5-HT agonist.
- With MAOIs (monoamine oxidase inhibitors) or if you have taken a MAOI in the last two weeks. Warnings and precautions
Talk to your doctor before using Sumatriptan Injection, if
- you have any of the following medical conditions: heart disease such as heart failure, angina or coronary thrombosis (heart attack), high blood pressure, disease of the liver or kidneys, epilepsy or brain disease (especially postmenopausal women and males over 40 years of age should have their heart and blood vessels checked before using this medicine),
- you have any risk factors for heart disease such as family history of heart disease; sugar diabetes; high blood cholesterol; if you are a regular cigarette smoker or if you are very overweight,
- you are allergic to certain antibiotics (sulphonamides); people allergic to sulphonamides may experience an allergic reaction to sumatriptan.
- you use certain medicines to treat a depression (a so called SSRI or SNRI) or lithium (a medicine used to treat manic/depressive (bipolar) disorders). You may develop the serotonin syndrome (including mental confusion, increased heart rate, shivering, sweating and muscle twitching). Ask your doctor for advice if you notice any of these symptoms.
After discussing the above, your doctor may still advise you to use Sumatriptan Injection and will instruct you about using the injection.
As with other migraine treatments, overuse could make your migraine worse and make them occur more often.
You may only use Sumatriptan Injection when your doctor is certain that you suffer from migraine headache or cluster headache.
Other medicines and Sumatriptan Injection
Before using Sumatriptan Injection tell your doctor if:
- you are taking any medicines for your migraine which contain ergotamine or ergotamine derivatives, such as ergotamine tartrate or methysergide maleate (if so, you should stop taking them at least 24 hours before using Sumatriptan Injection),
- you are taking any medicines on a doctor's prescription for the treatment of depression such as MAOI's or SSRI's (including citalopram, fluoxetine, fluvoxamine, paroxetine and sertraline), or if you have taken an MAOI in the last 2 weeks,
- you are taking lithium (a medicine used to treat manic/depressive (bipolar) disorders),
- you are taking any medicines on a doctor's prescription to help you lose weight, or for the treatment of epilepsy,
- you are taking anything containing the herbal remedy St John's Wort (Hypericum perforatum). Taking this together with Sumatriptan Injection may increase the likelihood of you suffering side effects.
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Ask your doctor or pharmacist for advice before taking this medicine
- if you are pregnant, think you may be pregnant or are planning to have a baby
_ - if you are breastfeeding. Your doctor may still advise you to use sumatriptan, but breastfeeding should
be avoided for 12 hours after a dose and during this time any breast milk expressed should be discarded.
Sumatriptan Injection may cause drowsiness. If you are affected do not drive or operate machinery. Sumatriptan Injection contains sodium
This medicinal product contains less than 1 mmol sodium (23 mg) per dose (i.e. 0.5 ml), i.e. essentially ‘sodium-free'.
Carefully read the ‘How to use the pre-filled pen’ section as provided at the end of this leaflet.
The pre-filled pen will inject a dose of Sumatriptan Injection just below the skin quickly and without pain. The injection must NOT be given in any other way than the way shown.
DO NOT inject Sumatriptan Injection into a vein.
DO NOT use Sumatriptan Injection to try and prevent an attack.
Use one pre-filled pen at the first sign of a migraine attack (although it will be equally effective if used at any time during an attack). If, after your first dose, your migraine goes away but then returns, you may use another pre-filled pen, provided it is at least an hour since the first injection. DO NOT use more than TWO injections in 24 hours.
If the injection does not ease your migraine, you may then take your usual 'pain killers', provided they do not contain ergotamine or its derivatives. Wait at least six hours after using Sumatriptan Injection before taking any medicines containing ergotamine or its derivatives.
If your migraine does not go, do not use a second one for the same attack. Sumatriptan Injection can be used for your next attack.
Use ONE pre-filled pen for each cluster attack. It should be used at the first sign of cluster headaches (although it will be equally effective if used at any time during an attack). DO NOT use more than TWO injections in 24 hours and make sure you leave at least one hour between the two doses.
Use in children and adolescents (under 18 years of age)
Sumatriptan Injection should not be used in children and adolescents under 18 years of age.
Use in elderly (over 65 years)
There is little experience of Sumatriptan Injection in those over 65 years of age so it is not usually prescribed for this age group.
If you use more Sumatriptan Injection than you should
Using more than prescribed may make you ill. If an overdose is used, DO NOT DELAY, ask your doctor what to do or contact your nearest accident and emergency department.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common( may affect more than 1 in 10 people)
- temporary pain at the place the injection was given
- stinging/burning, redness, swelling, bruising and bleeding at the place the injection was given.
Common (may affect up to 1 in 10 people)
- flushing (redness of the face lasting a few minutes), dizziness, feelings of weakness, tiredness and drowsiness
- short lasting increases in blood pressure soon after using the medicine
- feeling sick (nausea) or being sick (vomiting) - when not part of migraine attack
- pain, feelings of unusual sensations including tingling, numbness, heat or cold, heaviness and pressure or tightness. These symptoms usually pass quickly but may be intense and can affect any part of the body including the chest and throat. If these effects continue or are particularly severe, especially chest or heart pain which spreads to the arms, tell your doctor immediately as there have been rare reports of such problems being caused by a heart attack
- shortness of breath
- aching muscles (myalgia).
Very rare (may affect up to 1 user in 10,000 people)
- liver function changes: if you have a blood test to check how your liver is working and have used Sumatriptan, tell your doctor as it may affect the results.
Not known (frequency cannot be estimated from the available data)
- hypersensitivity reactions, which may vary from cutaneous reactions like rash, urticaria to anaphylactic reactions such as collapse
- seizures, tremors, muscle contractions, involuntary eye movements
- visual disturbances including flickering, double vision and reduced vision. There have been cases where permanent vision defects have occurred.
- lowering of blood pressure that can lead to feeling of faintness especially on standing up
- slowing or quickening in the speed of your heart beat, palpitations (feeling of fast heart beat), changes in heart rhythm
- Raynaud's phenomenon, which might appear as paleness or a blue tinge to the skin and/or pain of the fingers, toes, ears, nose or jaw in response to cold or stress
- heart attack
- chest pain (angina)
- ischaemic colitis with the following symptoms: abdominal pain, rectal bleeding and fever
- diarrhoea
- stiffness in the neck
- joint pain
- anxiety, sweating.
If you get the following side effects, you should contact your doctor immediately and do not use any more Sumatriptan unless your doctor tells you to do so.
- sudden wheeziness, fluttering or tightness in the chest, swelling of eyelids, face or lips, skin rash - red spots or hives (skin lumps), which may be signs of an allergic reaction
- fits (usually in people with a history of epilepsy)
- inflammation of the colon (part of the intestine), which may present as lower left-sided tummy pain and/or bloody diarrhoea
- Raynaud's phenomenon, which might appear as paleness or a blue tinge to the skin and/or pain of the fingers, toes, ears, nose or jaw in response to cold or stress
- chest pain (angina)
- heart attack.
If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme, website: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
375 mm
SUN EUROPE UK
100
95
75
25
5
0
Do not print grey area
5. How to store Sumatriptan Injection
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the pack. The expiry date refers to the
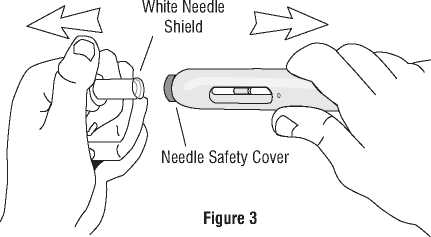
|ast day of that month. f) Pick up the pre-filled pen in one hand and smoothly remove the white needle shield by pulling it straight
off with your other hand. Do not twist it off, and do not recap the white needle shield, as either of these This medicinal product does not require any special storage conditions. may damage the needle inside the pre-filled pen.
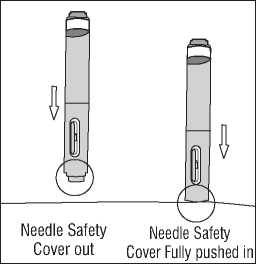
Needle Safety Cover flush with tip of barrel
Do not use this medicine if you notice any particles in the solution.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use, and how to dispose of the used pre-filled pens, or follow local instructions for disposal of the used pens. These measures will help to protect the environment.
6. Contents of the pack and other information What Sumatriptan Injection contains
- The active substance is Sumatriptan. Each pre-filled pen contains 6 mg of Sumatriptan, as sumatriptan succinate.
- The other ingredients are sodium chloride and water for injections.
What Sumatriptan Injection looks like and contents of the pack
Pre-filled pen, containing a clear colourless to pale solution for injection.
Each carton contains 1, 2 or 6 pre-filled pens.
Not all package sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Sun Pharmaceutical Industries Europe B.V. Polarisavenue 87 2132 JH Hoofddorp The Netherlands
+31-(0)23-5685501
+31-(0)23-5685505
This medicinal product is authorised in the Member States of the EEA under the following names:
Germany Sumatriptan-Hormosan Inject 6 mg/0,5 ml Injektionslosung
Denmark Sumatriptan SUN 12 mg/ ml injektionsraske, opl0sning
Spain Sumatriptan SUN 6 mg/0,5 ml solucion inyectable EFG
France Sumatriptan SUN 6 mg/0,5 ml solution injectable
Italy Sumatriptan SUN 6 mg/0,5 ml soluzione iniettabile
Netherlands Sumatriptan SUN 6 mg/0,5 ml oplossing voor injectie
Norway Sumatriptan SUN 12 mg/ml injeksjonsraske, oppl0sning
Sweden Sumatriptan SUN 12 mg/ml injektionsvatska, losning
United Kingdom Sumatriptan 6 mg/0.5 ml solution for injection
This leaflet was last revised in 06/2014
HOW TO USE THE PRE-FILLED PEN
Sumatriptan 6 mg/0.5 ml, Solution for Injection
This leaflet explains how to use the Sumatriptan pre-filled pen.
Read it TWICE before you begin the first step. If you have any questions,ask your doctor or pharmacist. Only for use in patients for whom a 6 mg dose has been prescribed.
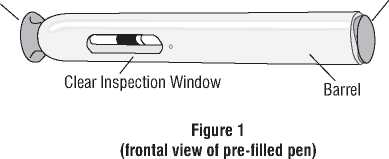
White Needle Shield Blue Activation Button
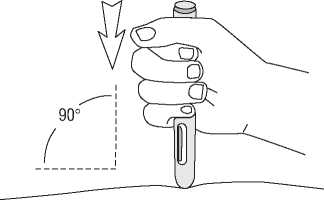
g) Without pressing the blue activation button, place the open end of the pre-filled pen on the injection site, straight up at a right angle (90°) and push the safety needle cover firmly against the skin to unlock. The pre-filled pen works only when the safety needle cover is fully retracted.
Pull Straight off
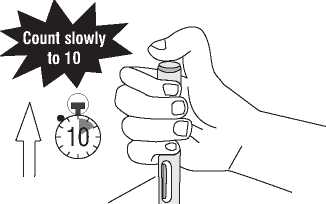
Continue to hold the pre-filled pen firmly against the skin.
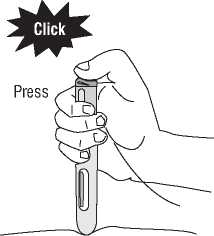
(1) Press the blue activation button (first click will sound). The blue activation button cannot be pressed if the safety needle cover is not fully retracted.
(2) This starts the injection.
(3) Do not lift the pre-filled pen off the skin. Hold down the button and count slowly to ten before you lift the pre-filled pen from the injection site.
(4) The inspection window will be blue, confirming the injection is complete.
(5) Lift the pre-filled pen straight up from the injection site. The injection is finished.
(6) Verify that the inspection window is blue to ensure that the injection is complete before lifting the prefilled pen.
If the inspection window is not blue, do not try to use the pre-filled pen again.
375 mm
Blue Inspection Window
Needle Safety Cover extends to cover needle
- Check the appearance of Sumatriptan , through the inspection window. It must be a clear, colorless to pale yellow solution. Do not inject the solution if it looks discolored or cloudy or contains lumps, flakes, or particles.
- Do not remove the white needle shield from the pre-filled pen until you are ready to inject.
(7) The safety needle cover on the pre-filled pen will automatically extend to cover the needle and lock into place. The needle will not be visible now.
There is no need to replace the white needle shield.
- NEVER put the white needle shield back into the pre-filled pen.
NEVER ATTEMPT TO REUSE A PRE-FILLED PEN.
- NEVER put or press thumb, fingers, or hand over white needle cover.
a) Wash your hands thoroughly.
b) Find a comfortable, well-lit place and put everything you need where you can reach it (pre-filled pen, alcohol or sterile swabs).
If you suspect you have not received the full dose, do not repeat the injection using a new pre-filled pen.
(8) If you notice a spot of blood at the injection site, dab away with a cotton ball or tissues. Do not rub the injection site. If needed, you may cover the injection site with a bandage.
c) Identify the application area with an adequate fatty tissue layer, for example on the upper arm or thigh. Do not inject into areas where the skin is tender, bruised, red, or hard.
d) Wipe the injection site with alcohol or a new sterile swab and allow your skin to dry. Do not touch this area again before giving the injection.
e) Take out the pre-filled pen from carton box.
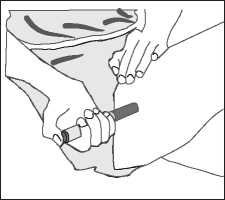
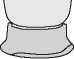
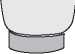
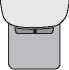
Figure 6
o
CO
r-~
r-~
51
CQ
^r
CD
Before Use (With White Needle Shield)
Before Use (Without White Needle Shield)
After Use (Needle Safety Cover down)
Q
O
9
a.
Q.
Z3
to
Folding_
375--3 --46.87 mm 248--1 --124 mm
248 mm
Size: 248x375 mm SUN EUROPE UK
100
95
75
25
5
0
Sumatriptan 6 mg-0-5 ml-EUUK-248x375mm-PIL-REV3-24-06-14
24 June 2014 19:19:29