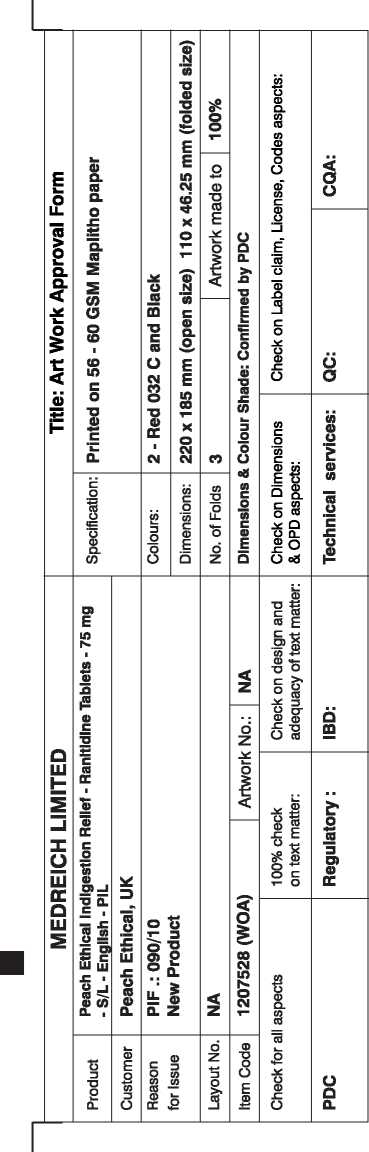
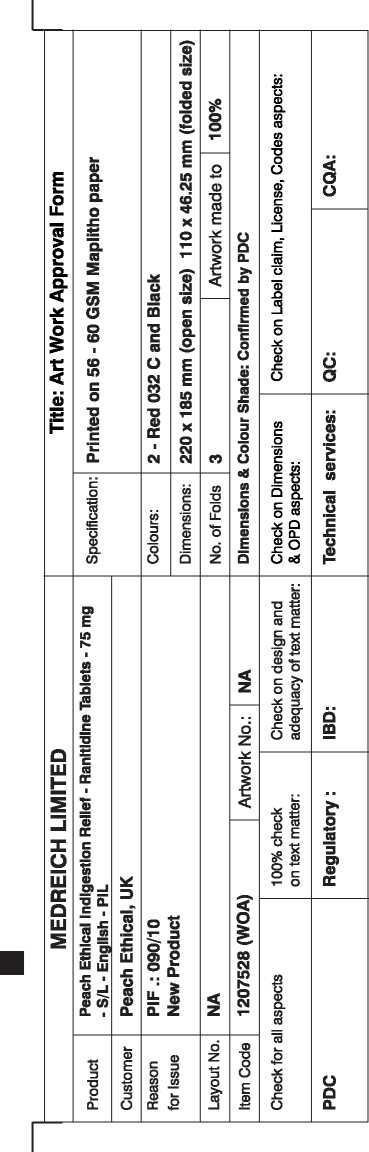
Superdrug Indigestion And Heartburn Relief Ranitidine 75Mg Film-Coated Tablets
Out of date information, search anotherJ

GSL
n
PL Holder:

MEDREICH pic
9, Royal Parade, Kew Gardens, Surrey TW9 3QD, England.
Red 032 C
1207528


Pharmacode No.: 2749
STORING RANITIDINE 75mg FILM-COATED TABLETS?
Keep out of the reach and sight of children. Never offer your medicine to other people. It may not be suitable for them even if their symptoms seem the same as yours.
| Do not store above 25°C.
Do not use after the expiry date stated on the label.
For further information about this medicine, please contact your nearest pharmacist or doctor.
PL No.: 21880/0022
This package leaflet was last revised in February 2010
®
Patient Information Leaflet Peach Ethical Indigestion Relief
RANITIDINE 75mg FILM-COATED TABLETS
ranitidine hydrochloride
Read all of this leaflet carefully before you start taking this medicine.
Keep this leaflet. You may need to read it again.
If you have further questions, please ask your doctor or your pharmacist.
In this leaflet:
1. What Ranitidine 75mg film-coated tablets are and what these are used for?
2. Before you take Ranitidine 75mg film-coated tablets?
3. How to take Ranitidine 75mg film-coated tablets?
4. Possible side effects?
5. Storing Ranitidine 75mg film-coated tablets?
Ranitidine 75mg film-coated tablets come in packs of 6 and 12 tablets.
Each 75mg tablet contains Ranitidine hydrochloride equivalent to 75mg of Ranitidine
- The other ingredients are: Microcrystalline cellulose.
Magnesium stearate, Hypromellose, Titanium dioxide (El 71).
WHAT RANITIDINE FILM-COATED TABLETS ARE AND WHAT THESE ARE USED FOR?
Your medicine is known as a histamine H2 antagonist. It works by reducing the natural production of acid in the stomach.
Ranitidine film-coated tablets are used for:
Symptomatic relief of heartburn, indigestion, acid indigestion and hyperacidity.
The product is not indicated in the following people without seeking their doctor's advice:
Those with difficulty swallowing, persistent stomach pain or unintended weight loss in association with symptoms of indigestion. Those who are middle-aged or elderly with new or recently changed symptoms of indigestion.
BEFORE YOU TAKE RANITIDINE 75mg FILM-COATED TABLETS?
Do not take RANITIDINE 75mg FILM-COATED TABLETS:
If you are hypersensitive (allergic) to ranitidine or any of the other ingredients of RANITIDINE 75mg FILM-COATED TABLETS.
MED/RAN/PIL/06 WIN/Artworks/Peach Ethical/Ranitidine/Tablets/75 mg/PIL/1207528.ai 22/23.02.10(SK)/17.03(MA)/
Format No.: CO-QA005-F06-00 Internal Approval Issued on : 17.05.10 (GW) CD Released on 09(MA)/26.04(MA)/17.08.10(SK)
J


n


Take special care with RANITIDINE 75mg FILM-COATED TABLETS:
If you answer YES to any of the following questions, DO NOT take this medicine until you have talked to your doctor. You may need | to be given a different medicine orthe dose may need to be changed.
Do you have a kidney problem?
Do you have a blood disease?
Have you had a stomach ulcer or a duodenal ulcer before? You should discuss this with your doctor particularly if you are taking a non-steroidal anti-inflammatory drug (NSAID).
Pregnancy:
Like other over the counter drugs Ranitidine 75mg Tablets should not be taken during pregnancy without consulting a doctor or pharmacist.
Breast-feeding:
Since Ranitidine is excreted in human breast milk, breast-feeding women should speak to their doctor before taking these tablets.
Driving and using machines:
Not applicable.
Using other medicines:
Please inform your doctor or pharmacist if you are taking or have recently taken any other medicines, even those not prescribed.
HOW TO TAKE RANITIDINE 75mg FILM-COATED TABLETS?
Adults (Including the elderly) and children 16 years of age and older:
Swallow one Ranitidine 75mg film coated Tablet whole, with a drink of water, as soon as you have symptoms. If symptoms persist for more than one hour or return, take another tablet. Do not take more than two tablets in 24 hours.
Do not take the tablets for more than 6 days without the advice of a pharmacist or doctor.
Children under 16 years:
Not recommended for children under 16 years of age.
@ 120752
If you take more Ranitidine 75mg film-coated tablets than you should:
Ranitidine is very specific in action and accordingly no particular problems are expected following overdosage with the drug. Symptomatic and supportive therapy should be given as appropriate. If need be, the drug may be removed from the blood by haemodialysis.
If you forget to take Ranitidine 75mg film-coated tablets:
Do not take a double dose to make up for forgotten individual doses. Take your next dose at the normal time.
POSSIBLE SIDE EFFECTS
Like all medicines, Ranitidine 75mg film-coated tablets can have side effects.
Side effects with your medicine are usually mild and do not last long. The most common unwanted effects are diarrhoea, dizziness, rash and tiredness.
Very rarely, jaundice (yellowing of the skin or the whites of your eyes) and pancreatitis may occur.
Blood abnormalities, allergy, mood changes, confusion, psychoses or hallucinations, pain in muscles and joints, slow heartbeat and heart block (which can cause dizziness or fainting) are possible.
A few people can be allergic to some medicines; if any of the following side effects come on soon after taking the tablets, STOP the tablets and tell your doctor immediately:
- sudden wheezing or tightness in the chest;
- swelling of eye lids, face or lips; with or without a lumpy skin rash ("hives") anywhere on the body;
unexplained fever;
- feeling faint, especially on standing up.
Do not be alarmed by this list of side effects. You may not have any of them.
If you notice any side effects not mentioned in this leaflet, please inform your doctor or pharmacist.
MED/RAN/PIL/06 WIN/Artworks/Peach Ethical/Ranitidine/Tablets/75 mg/PIL/1207528.ai 22/23.02.10(SK)/17.03(MA)/
Format No.: CO-QA005-F06-00 Internal Approval Issued on : 17.05.10 (GW) CD Released on 09(MA)/26.04(MA)/17.08.10(SK)
