Survanta 25Mg/Ml Suspension
• If any vial is not used within 8 hours of re-warming to room temperature it should be thrown away. Vials should not be returned to the refrigerator once warmed.
Medicines should not be disposed of via wastewater or household waste.
6. Contents of the pack and other information
What Survanta contains
-The active substance is beractant which is a mixture containing phospholipids (25 mg/ml), free fatty acids (1.4 -3.5 mg/ml), triglycerides (0.5 -1.75 mg/ml) and protein (0.1 -1.0 mg/ml).
-The other excipients are sodium chloride, sodium hydroxide, hydrochloric acid, palmitic acid, dipalmitoyl phosphatidylcholine, tripalmitin and water.
What Survanta looks like
-It is a sterile off-white to light brown suspension and is supplied in a single use glass vial containing 8 ml (200 mg phospholipids). Packs of 1,3, and 10 vials are available.*
A
IMPORTANT
INFORMATION
Read all of this leaflet carefully before this medicine is given because it contains important information for you.
What is in this leaflet
1. What Survanta is and what it is used for
|
abbvie (Prepared by AbbVie Graphics) | |
|
LIST NO. |
1039-53 |
|
COMMOD. NO. |
03-B190 |
|
PPLS VERSION |
1 |
|
cr no. 0001146-2015 | |
|
LABEL EDITOR |
Dorr |
|
DATE PREPARED |
8/5/15 |
|
DRAWING NUMBER |
SB-6010-0 |
|
CYCLE |
1 |
|
ARTIST |
BO |
AbbVie Label Control Approval
Approved by
Date
Not valid unless final proofs carry Label Control Approval signature
Color Separations
PMS Black
PMS2768 C
PMS 185 C
*Not all pack sizes may be marketed.
Marketing Authorisation Holder:
AbbVie Ltd.,
Maidenhead, SL6 4UB. UK
Manufacturer:
AbbVie Logistics B.V. Zuiderzeelaan 53 8017 JV Zwolle The Netherlands
This leaflet was last revised in:
July 2015
List 1039-53
2. What you need to know before Survanta is used
3. How to use Survanta
4. Possible side effects
5. How to store Survanta
6. Contents of the pack and other information
1. What Survanta is and what it is used for
|
1 1 | ||
|
1 1 1 | ||
|
03-B190 | ||
Survanta contains the active substance beractant which is a natural surfactant extracted from cow’s lungs (see section 6) to help your child breathe.
Your baby will be/has been given Survanta because he or she is at risk of developing, or is suffering from, a condition called Respiratory Distress Syndrome (hyaline membrane disease) which may cause severe breathing difficulties.
Survanta is indicated for treatment of Respiratory Distress Syndrome (RDS) in newborn premature infants with a birth weight of 700 g or greater and who have had tube inserted and are on a mechanical ventilator to help them breathe.
Survanta is also used for the treatment of premature babies, when the pregnancy has lasted for less than 32 weeks, at risk of developing RDS.
Respiratory Distress Syndrome occurs in some babies, particularly premature babies, who lack a substance usually produced in the lungs known as surfactant. This surfactant lines the inside of the lungs, stopping them from sticking together, so that the baby can breathe normally.
Survanta as a natural surfactant acts in a similar way to your baby’s own surfactant helping your baby to breathe normally.
4. Possible side effects
2. What you need to know before Survanta is used
Your baby will only be given Survanta if the equipment for ventilation and monitoring babies with Respiratory Distress Syndrome is available.
After being given Survanta, your baby will continue to be monitored by the doctor or nurse to ensure that the right amount of oxygen is being given.
During the dosing procedure, occasional episodes of slow heartbeat (bradycardia) and/ or oxygen reduction in the circulation have been reported. If these occur, dosing will be stopped and appropriate measures to relieve the condition will be started. After stabilisation, the dosing procedure will be resumed.
3. How Survanta is used
The dosage of Survanta varies for each child depending on their body weight. The usual dose is 100 mg Survanta per kg body weight. The doctor will calculate the right dose. Usually the first dose will be given as soon as possible after birth (usually within 15 minutes) or as soon as possible after Respiratory Distress Syndrome has been diagnosed (usually within 8 hours of birth).
The dose of Survanta will be administered to your baby via a tube already in place in your baby’s windpipe. Do not be concerned if your baby is disconnected from its ventilator while Survanta is being administered. To make sure that Survanta reaches all parts of your baby’s lungs, the dose is split into smaller doses and your baby’s position altered before each part of the dose is given.
The dose may be repeated up to three times at six hourly intervals within 48 hours. Survanta will be warmed to room temperature before administration to your baby.
Like all medicines, Survanta can be associated with side effects although not everybody gets them.
The following side effects with Survanta are serious and will be managed by your baby’s Doctor as necessary during dosing.
Very common: affecting more than 1 in 10:
• Bleeding in the brain. The occurrence of this side effect is no different to what would be expected in untreated babies of the same age.
Common: affecting less than 1 in 10
• Cases of bleeding in the lungs.
Other Side effects:
Uncommon: affecting less than 1 in 100
• Blockage of the breathing tube that has been inserted into your baby’s windpipe.
If you have any questions about your baby’s treatment which are not answered by this leaflet, ask the doctor.
Reporting of side effects
If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly (see details below). By reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
Malta
ADR Reporting
www.medicinesauthority.gov.mt/
adrportal
5. How to store Survanta
Keep out of the sight and reach
of children.
• Survanta should not be used after the expiry date shown on the label.
• Survanta should have been stored in a refrigerator and protected from light; however before it
is given to your baby it will be warmed to room temperature.
• Survanta must not be frozen.
Any product that has been frozen by mistake should be thrown away.
• Each vial of Survanta is for single use only. Used vials with medicine left in them should be thrown away.
|
abbvie (Prepared by AbbVie Graphics) | |
|
LIST NO. |
1039-53 |
|
COMMOD. NO. |
03-B190 |
|
PPLS VERSION |
1 |
|
cr no. 0001146-2015 | |
|
LABEL EDITOR |
Dorr |
|
DATE PREPARED |
8/5/15 |
|
DRAWING NUMBER |
SB-6010-0 |
|
CYCLE |
1 |
|
ARTIST |
BO |
AbbVie Label Control Approval
Approved by
Date
Not valid unless final proofs carry Label Control Approval signature
Color Separations
PMS Black
PMS2768 C
PMS 185 C
2
3
L6 Lg-co
Description and Composition
Survanta is a sterile, non-pyrogenic pulmonary surfactant intended for intratracheal use only.
It is a bovine lung extract containing phospholipids, neutral lipids, fatty acids and surfactant associated proteins to which have been added dipalmitoyl phosphatidylcholine, palmitic acid and tripalmitin.
The resulting composition provides:
phospholipids 25 mg/ml (including 11.0 - 15.5 mg/ml disaturated phosphatidylcholine) triglycerides 0.5 - 1.75 mg/ml free fatty acids 1.4 - 3.5 mg/ml protein less than 1.0 mg/ml It is suspended in 0.9% sodium chloride (Ph Eur) solution. It also contains sodium hydroxide and hydrochloric acid.
Each millilitre of Survanta contains 25 mg phospholipids. It is an off-white to light brown liquid supplied in single-use glass vials containing 8 ml (200 mg phospholipids). Properties
Endogenous pulmonary surfactant lowers surface tension on alveolar surfaces during respiration and stabilises the alveoli against collapse at resting transpulmonary pressures. Deficiency of pulmonary surfactant causes Respiratory Distress Syndrome (RDS) in premature infants. Survanta replenishes surfactant and restores surface activity to lungs of these infants.
Therapeutic Indications Survanta is indicated for the treatment of Respiratory Distress Syndrome (RDS) in newborn premature infants with birth weight of 700 g or greater who are intubated and receiving mechanical ventilation. Survanta is also indicated for the prophylactic treatment of premature infants <32 weeks gestational age at risk of developing RDS. Contraindications
No specific contraindications for Survanta have been defined by the clinical studies. Warnings and Precautions Special Precautions for Use:
Survanta should only be administered with adequate facilities for ventilation and monitoring of babies with RDS.
Marked improvements in oxygenation may occur within minutes of the administration of Survanta. Therefore, frequent and careful monitoring of systemic oxygenation is essential to avoid hyperoxia. Following Survanta administration, monitoring of the arterial blood gases, the fraction of inspired oxygen and ventilatory change is required to ensure appropriate adjustments.
During the dosing procedure, transient episodes of bradycardia and/or oxygen desaturation have been reported. If these occur, dosing should be stopped and appropriate measures to alleviate the condition should be initiated. After stabilisation the dosing procedure should be resumed.
Survanta is stored refrigerated (2-8°C). Before administration, Survanta should be warmed by standing at room temperature for 20 minutes or warmed in the hand for 8 minutes. ARTIFICIAL WARMING METHODS SHOULD NOT BE USED.
Discard each vial if not used within 8 hours of warming to room temperature. Vials should not be returned to the refrigerator once warmed.
Each vial of Survanta is for single use. Used vials with residual drug should be discarded. Survanta should be inspected visually for discolouration prior to administration. The colour of Survanta is off-white to light brown. Some settling may occur during storage. If this occurs, gently invert the vial several times (DO NOT SHAKE) to redisperse.
Interactions with Other Medications
None known to date.
Dosage Instructions
Unless otherwise prescribed, 4 ml suspension per kg birth weight is the normal dose to be administered via the trachea (intratracheal administration).
Higher doses should not be used.
SL0£ A|nr Uj P0SJA0J JSB| SBM }0|JB0| SjL|X
>in ant7 9is
'pB0i|U0pjB|/\| :jap|°H
' pj“| 0jAqqV uonesuoi|inv 6uua>jJB
:(ei|ir ) jaqiimN L0E00/7ES VIAI uoijesuoiunv 6mio>|je|/\|
:()in)Jaqiun|\|
£000/21r0l1r1d aouaon lonpojj
lAIOd :AjoBo(03 |e6aq
UMOjq jq6|| oj aj|qM-jjo s| ejueAjng jo jno|oo 9i|X uo|jejjs|U|uipe oj joud uo|jejno|oos|p joj A||Bns|A pajoadsm aq p|noqs ejueAjng papjBosjp aq p|noqs jonpojd uazojj A|jU0jj0ApBUj Au\/ 'dZdddd TON 00 0o 8 -11® uojjBJofiNjoj japun pajojs pus jqBq uiojj psjosjojd 0q jsnui ejueAjng jo posodsjp
oq p|noqs ejueAjng jo sjunouiB 6u|u|euiay '(3J.Bp Ajjdxo JOJ |0qB| |B|A 90S) 0np S||BJ 0JBp Ajidxa aq; jsjjb pasn aq jou p|noqs Bmp siqx suojjneoajy leounaoeiujeqy juasajd aje uoqonjjsqo Abmjib jo suBis jno-jB0|o ssa|un pajinbaj jou si uoijob |B|pauiaj jaqjo jo Buiuoqons |BaqoBjj-opug aBssopjaAO ajBOipUl JOU Op pUB 'U0AI6 SI BJUBAjng J0JJB jnooo ApuaisuBj; ubo spunos qjsajq jsioui pus sa|By aAtjjoddns pus oijBuiojduiAs aq pInoqs JU0WJB0JX uoijonjjsqo Abmjib ajnoB jo suBis joj jubjui aq; aAjasqo 'usaiB si BjuBAjns jo asop 06jb| A|9Aissaoxa ub j|
asopjaAO
|BJJOdjpB
/juiAoBAjiJoqjnBsauioipaujMMM:atisqa/y\ Buqjoday yg\/ 0)|0|/\|
pjBOMO||aA/)|n AoB Bjquj MMM :ajisqa/y\
:auiaqos pjsg Moya^ iuopBuj)| pajmfi
:ojjoajip
suoijoBaj asjaApB pajoadsns Aub jjodaj oj paqsB ajB s|Buoissajojd ajBoqj|Bay jonpojd |Buioipaui aq; jo aouB|Bq qsij/jijauaq aq; jo Buijojiuoui panuijuoo smo||b j| juBjjodun si jonpojd |Buioipaui aqj jo uoijBsuoqjnB jajjB suoijoBaj asjaApB pajoadsns Buqjoday suojjaeaj asjaApe pajoadsns jo Buqjoday
paAjasqo uaaq ssq suiajojd BjuBAjns oj uoijonpojd Apoqijus o|\|
(00L/L> '000T/l<) uouiiuooun f(0L/L> '00l/l<) UOUIIUOO f(0L/L<) UOUIIUOO Aja/\ :pasn ajB sauoBajBO Aouanbajj Buimo||oj aqx
|
SUOJJ0JO0S snoonui Aq 0qnj |B0qOBJJOpU0 jo 06B>|3O|g |
110IU LUO 3111"| |
sojnpooojg |B3Jp31/\| puB |B3j6jng |
|
aBBqjjouiasq AjBuouqny |
110 111 111 OQ |
AjojBj|dsay |
|
06BqjJOW0Bq |BjUBJOB4U| |
UOIUIUOO AJ9A |
SJ0pJOS|p JB|n3SB/\ |
|
suouoBay 3SJ3APV |
Aouanbajj |
ssB|Q uefjjj) uiajsAs |
:a|qBj Buimo||oj aqj ui pajuasajd ajB asaqx
|
abbvie (Prepared by AbbVie Graphics) | |
|
LIST NO. |
1039 (53) |
|
COMMOD. NO. |
03-B191 |
|
PPLS VERSION |
1 |
|
CR NO. |
0001146-2015 |
|
LABEL EDITOR |
Dorr |
|
DATE PREPARED |
8/7/15 |
|
DRAWING NUMBER |
SB-5995-0 |
|
CYCLE |
1 |
|
ARTIST |
BO |
AbbVie Label Control Approval
Approved by
Date
Not valid unless final proofs carry Label Control Approval signature
Color Separations
PMS Color
The dosing chart below shows the total dosage for a range of birth weights.
|
Weight (grammes) |
Total Dosage (ml) |
Weight (grammes) |
Total Dosage (ml) |
|
701 - 750 |
3.0 |
1351 - 1400 |
5.6 |
|
751 - 800 |
3.2 |
1401 - 1450 |
5.8 |
|
801 - 850 |
3.4 |
1451 - 1500 |
6.0 |
|
851 - 900 |
3.6 |
1501 - 1550 |
6.2 |
|
901 - 950 |
3.8 |
1551 - 1600 |
6.4 |
|
951 - 1000 |
4.0 |
1601 - 1650 |
6.6 |
|
1001 - 1050 |
4.2 |
1651 - 1700 |
6.8 |
|
1051 - 1100 |
4.4 |
1701 - 1750 |
7.0 |
|
1101 - 1150 |
4.6 |
1751 - 1800 |
7.2 |
|
1151 - 1200 |
4.8 |
1801 - 1850 |
7.4 |
|
1201 - 1250 |
5.0 |
1851 - 1900 |
7.6 |
|
1251 - 1300 |
5.2 |
1901 - 1950 |
7.8 |
|
1301 - 1350 |
5.4 |
1951 - 2000 |
8.0 |
Method of Administration
Survanta should be administered by intratracheal instillation (ie: drug should be conducted into the lungs via an endotracheal tube) using a 5 Fr catheter. The tip of the catheter should lie at the end of the endotracheal tube. Infants should not be intubated solely for the administration of Survanta.
Survanta should be warmed to room temperature before administration (see Special Precautions for Use).
Before administering Survanta in infants on mechanical ventilation, set the respiratory frequency at 60/minute - with inspiration time 0.5s and Fi02 at 1.0. Inspiratory pressure needs no change at this point.
To ensure distribution of Survanta throughout the lungs, each dose is divided into fractional doses. Each dose can be administered as either two half-doses or four quarter-doses. Each fractional dose is administered with the infant in different positions as given below. Between each position the infant should be ventilated for 30 seconds.
For Four quarter-doses, the recommended positions are:
Right Lateral Position with the head lowered (i.e. head and body slanting down at an angle of approximately 15°).
Left Lateral Position with the head lowered (i.e. head and body slanting down at an angle of approximately 15°).
Right Lateral Position with the head elevated (i.e. head and body slanting up at an angle of approximately 15°).
Left Lateral Position with the head elevated (i.e. head and body slanting up at an angle of approximately 15°).
For administration of each quarter dose, the ventilator is disconnected, the catheter inserted, the dose administered then the ventilator reconnected.
Between each quarter dose the infant is ventilated for 30 seconds.
See illustrations below for recommended position
Right lateral position
with the head lowered, i.e., head and body slanting down.
Left lateral position
with the head lowered, i.e. head and body slanting down.
pajjodaj uaaq aABq suoijoBaj asjaApB snoijas jaqjo o|\| pajjodaj uaaq ssq suoijajoas snoonui Aq aqnj |BaqoBjjopua aqj jo aBBqooiy pajjodaj uaaq os|B ssq aBsqjjoujaBq AjBuoui|ny uoijB|ndod juaijsd siqj ui ajnjBjajq aqj ui pajjodaj jsqj oj jb|iuiis si sjuaijed ye ui aBeqjjouiaeq Ib|ubjobjju| jo aouapioui aqx oqaoB|d jo juBjoBjaq jaqjia paAiaoaj oqM sjuaijsd ui paAjasqo uaaq ssq aBBqjjouiaBq |biubjobjju| sjoajjd a|qej;sapun sjnoq 8f7 jsjq aqj ui usaiB aq Aeui sasop |BuoijippB £ oj df| asop snoiAajd aqj jsjjb sjnoq 9 ueqj jauoos ou asoQ ssajjsip AjojBJidsaj Buinuijuoo jo aouapiAa Aq pauiuuajap aq p|n0qs BjuBAjng jo sasop |BuoijippB joj paau aqi jqBiaM qjjiq s.jubjui aqj uo paseq si puB Bq jad spidi|oqdsoqd Bui ooi 0SIB s! BjuBAjng jo sasop jsadaj joj aBssop aqx
sajnuiui gj uiqjiMA|qBjajajd 'qjjiq jsjjb aiqissod sb uoos sb pajajsiuiuipB aq p|noqs BjuBAjng jo asop jsjij aqx :sixB|Aqdojy
aBs jo
sjnoq 8 Aq A|qbjajajd 'ggy jo aouajjnooo jsjjb aiqissod sb uoos sb paqddB aq p|noqs BjuBAjng jo asop jsjij aqx ijuauijsajx
BjuBAjng qj|M Buisop aqj Buiqsiuij jsjjb A|ajBipauiuii sjajauiBJBd Ajojb|iju9a ui saBusqo joj qoaqo pus 'uoijBjjuaouoo uaBAxo AjojBJidsui aqj pus san|BA sbB poo|q |bij9jjb |Ojjuog
A|aja|duioo UMBjpqjiM uaqj si jajaqjso aqX pajajsiuiuipB asop j|bq puooas aqj pus aqnj |BaqoBjjopua aqj ojui pajjasuiaj uaqj si jajaqjso aqx jojoauuoo aqj uiojj paAouiaj jou jnq aqnj |BaqoBjjopua aqj uiojj pajoBjjaj si jajaqjso aqj auiij qoiqM Buunp sasop j|Bq aqj uaaMjaq spuooas 0£ jsb9| jb aq p|noqs ajaqx JojB|ijuaA aqj uiojj uoijoauuoosip jnoqjiM jojoauuoo jjod uoijons b qBnojqj jajaqjso aqj Buijjasui Aq pajajsiuiuipB si asop j|Bq jsjij aqx
(jojoauuoo jjod uoqons b qBnojqj) jojB||juaA aqj uiojj uo|joauuoos|p jnoqj|M uo|jB|||jsu| 'A|9A|JBUjaj|\/ spuooas 0£
joj pajoauuooaj s| jojB||juaA aqj 'sasop j|Bq aqj uaaMjag asop j|Bq aqj Buuajs|U|uipB pus jajaqjso aqj Bu|jjasu| 'jojBpjuaA aqj uiojj aqnj |BaqoBjjopua aqj Buqoauuoos|p Aq pajajs|U|uipB s| asop j|bq qosg
J0JB||JU9A
aqj uiojj uo|joauuoos|p qj|M uo|jB|||jsu|
:UO|JBJJS|U|UipB jo spoqjaui 9A|jBUjaj|B 1 sjb ajaqj pajajsm|uipB Burnq sjb BjuBAjng jo sasop-j|Bq oaaj uaq/y\
|
r \ | ||
■ jjaI aqjojcgj7 A|ajBui|xojddB paujnj Apoq pus psaq aqj 'au|dns jubju| aqj qj|/y\
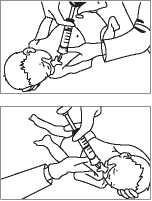
jqB|j aqjojcgj7 A|ajBui|xojddB paujnj Apoq pus psaq aqj 'au|dns jusjm aqj qj|/y\
:ajB suoq|sod
papuauiuiooaj aqj ‘sasop-j|ei| oaax joj
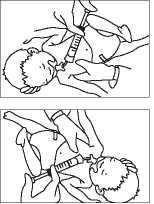
dn Bu|jub|s Apoq pue peaq 'at 'pajBAa|a peaq aqjqj|M uo;j;sod |BJ3je| jjaq
dn Bu|jub|s Apoq pue peaq at 'pajBAa|a psaq aqj qj|M uo;j;sod |BJ3je| ji|h;y