Tapclob 10Mg/5Ml Oral Suspension
PACKAGE LEAFLET: INFORMATION FOR THE USER
Clobazam
o
LrsJ
O
■P"
■P"
JCD
P
P
O
o
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again
• If you have any further questions, ask your doctor, nurse or pharmacist.
• This medicine has been prescribed for you.
Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
1. What Tapclob® Oral Suspension is and what it is used for
2. What you need to know before you take Tapclob® Oral Suspension
3. How to take Tapclob® Oral Suspension
4. Possible side effects
5. How to store Tapclob® Oral Suspension
6. Contents of the pack and other information
1. What Tapclob® Oral Suspension is and what it is used for
Tapclob® Oral Suspension contains clobazam which belongs to a group of medicines called benzodiazepines.
Clobazam works by having a calming effect on the brain.
Tapclob® Oral Suspension is used to treat:
Severe anxiety over a short time
Epilepsy (fits) over a longer time (in combination with
other treatments)
Mental illness such as schizophrenia (in combination with other treatments)
2. What you need to know before you take Tapclob® Oral Suspension
Do not take Tapclob® Oral Suspension:
If you are allergic (hypersensitive) to clobazam, other benzodiazepine medicines or any of the other ingredients of this medicine (listed in section 6).
If you are in the first three months of pregnancy or think you might be pregnant (see 'Pregnancy and breastfeeding' for more information).
If you are breast-feeding.
If you have ever had problems with drugs or alcohol dependence in the past.
If you suffer from an illness that causes muscle weakness (called 'myasthenia gravis1).
If you have severe liver problems.
If you have breathing problems.
I f you stop breathing for short periods during sleep (called 'sleep apnoea syndrome').
Warnings and precautions
Talk to your doctor before taking Tapclob® Oral Suspension: If you have problems with controlling your movements (called 'spinal or cerebellar ataxia').
If you have depression, irrational fears and obsessions.
If you have delusions (believing things which are not true) or hallucinations (sensing things which are not there).
If your liver or kidneys do not work as well as they should.
■ If you have ever become dependent upon another drug or alcohol.
Suicidal thoughts
Some patients have experienced suicidal thoughts while taking medicines containing clobazam, particularly if they are already depressed. If you start experiencing thoughts of suicide or harm towards yourself please tell your doctor immediately.
Dependence, tolerance and withdrawal
t is possible for you to become dependent on Tapclob® Oral Suspension if you take it for long periods of time, particularly if you have a history of heavy alcohol or drug use. This means that you may feel that you need to continue treatment with Tapclob® Oral Suspension in order to feel well (known as psychological dependence). f you suddenly stop taking Tapclob® Oral Suspension you may experience worsening of the symptoms you were originally being treated for, as well as mood changes, anxiety, sleep disturbance or restlessness. This is known as withdrawal and can be avoided by slowly reducing your dose. If you are worried about dependence or withdrawal please talk to your doctor.
f you take Tapclob® Oral Suspension for long periods of time for treatment of epilepsy it is possible that you may become tolerant to it, meaning that it will not be as effective as it was when you first started taking it. If you feel that Tapclob® Oral Suspension is no longer helping to control your symptoms please talk to your doctor, they may suggest you take a short break from this medicine.
Other medicines and Tapclob® Oral Suspension Please tell your doctor, pharmacist or nurse if you
are taking, have recently taken or might use any other medicines, including medicines obtained without a prescription. Especially: medicines for epilepsy (such as phenytoin, carbamazepine or valproic acid) medicines for depression (such as MAOIs, SSRI's or tricyclic anti-depressants - such as trazodone) medicines for severe mental illness called 'neuroleptics' (such as chlorpromazine, haloperidol and clozapine) painkillers (such as medicines containing codeine, dihydrocodeine or morphine) sleeping tablets (such as zolpidem) tranquilisers (such as diazepam, temazepam or lorazepam)
muscle relaxants (such as baclofen) antihistamines that make you sleepy (such as chlorphenamine, promethazine or diphenhydramine) lithium - used for a mental illness called 'bipolar disorder (mood changes between a state of high excitability or exaggerated emotions and depression) cimetidine (used to treat ulcers and heartburn)
Anaesthetics
f you are going to have an anaesthetic, tell your doctor or anaesthetist you are taking Tapclob® Oral Suspension. This s because your doctor may need to change the amount of anaesthetic or muscle relaxants given to you.
Tapclob® Oral Suspension with food, drink and alcohol Do not drink alcohol while you are taking Tapclob® Oral Suspension. This is because alcohol can change the way Tapclob® Oral Suspension works.
Pregnancy and breast-feeding
Do not take Tapclob® Oral Suspension if you are in the first three months of pregnancy are breast-feeding
f you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor ,:or advice before taking this medicine. Tapclob® Oral Suspension should be avoided during pregnancy.
f you take Tapclob® Oral Suspension late in pregnancy or during labour your baby might have a low body temperature, floppiness, breathing or feeding difficulties. f taken regularly during late pregnancy, your baby may develop withdrawal symptoms.
Driving and using machines
You may feel sleepy or have concentration or memory problems after taking this medicine.
You may also experience double vision or you may react more slowly to things. Do not drive or use any tools or machines if you are affected in this way.
The medicine can affect your ability to drive as it may make you sleepy or dizzy.
Do not drive while taking this medicine until you know how it affects you.
It is an offence to drive if this medicine affects your ability to drive.
However, you would not be committing an offence if:
• The medicine has been prescribed to treat a medical or dental problem and
• You have taken it according to the instructions given by the prescriber or in the information provided with the medicine and
• It was not affecting your ability to drive safely
Talk to your doctor or pharmacist if you are not sure whether it is safe for you to drive while taking this medicine.
Tapclob® Oral Suspension contains:
Sorbitol (E420): If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product Sodium methyl hydroxybenzoate (E219) and sodium propyl hydroxybenzoate (E217): These may cause allergic reactions in some people that could occur some time after taking this medicine. The signs may include a rash, swallowing or breathing problems and swelling of your lips, face, throat or tongue
f you have any concerns over whether this medicine is suitable for you talk to your doctor, pharmacist or nurse.
3.How to take Tapclob® Oral Suspension
Always take this medicine exactly as your doctor has told you. Tapclob® Oral Suspension is usually given for 2 to 4 weeks. After that, your doctor will decide whether you should keep taking this medicine. Check with your doctor or pharmacist if you are not sure.
When you are taking Tapclob Oral Suspension you should not change to any different clobazam containing medicines except under your doctor's supervision.
5mg/5ml: Doses
Adults and adolescents (over 15 years)
The usual dose is 20mg (20ml) to 30mg (30ml) each day. This can be taken as two separate doses or as a single dose at night.
Your doctor may increase your dose to up to 60mg (60ml) each day.
Your doctor may lower the dose to suit you.
Children (over 6 years)
The usual starting dose is 5mg (5ml) each day.
Your doctor will then adjust the dose according to your child's need.
Elderly
The usual dose for anxiety is 10mg (10ml) to 20mg (20ml) each day.
10mg/5ml: Doses
Adults and adolescents (over 15 years)
The usual dose is 20mg (10ml) to 30mg (15ml) each day. This can be taken as two separate doses or as a single dose at night.
Your doctor may increase your dose to up to 60mg (30ml) each day.
Your doctor may lower the dose to suit you.
Children (over 6 years)
The usual starting dose is 5mg (2.5ml) each day.
Your doctor will then adjust the dose according to your child's need.
Elderly
The usual dose for anxiety is 10mg (5ml) to 20mg (10ml) each day.
Method of administration:
This product may settle during storage, please shake well before use.
Your doctor, pharmacist or nurse will show you how to administer this medicine. The box containing this medicine will contain a 5ml dosing syringe, a dosing adaptor and a 30ml dosing cup.
5ml syringe each numbered increment is 1ml equivalent to 1mg of Tapclob 5mg/5ml Oral Suspension and 2mg of Tapclob 10mg/5ml Oral Suspension . The smaller increments are 0.2ml or 0.2mg of Tapclob 5mg/5ml Oral Suspension and 0.4mg of Tapclob 10mg/5ml Oral Suspension.

30ml dosing cup. Each numbered increment is 5ml -equivalent to 5mg of Tapclob 5mg/5ml Oral Suspension and 10mg of Tapclob 10mg/5ml Oral Suspension.
Instructions are provided overleaf for using the dosing syringe. If you have any questions about the dose you should use or how to use the syringe, you should ask your pharmacist.
Continued overleaf
3044-H
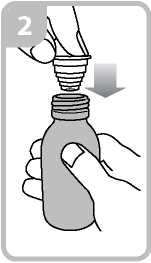
• Holding the bottle, take the plastic syringe adaptor from the box and insert the adaptor into the bottle neck (figure 2).
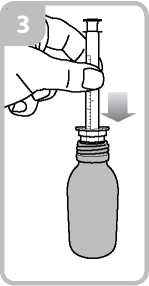
• Take the syringe and put it in the adaptor opening (figure 3).
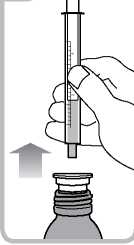
• Turn the bottle the right way up. Remove the syringe from the adaptor
(figure 5).
6
nstructions for use:
• Open the bottle: press the cap and turn it anticlockwise (figure 1)
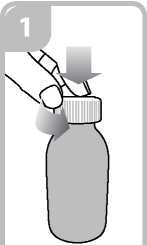
Vi
Ensure it is well fixed.
• Turn the bottle upside down.
• Fill the syringe with a small amount of suspension by pulling the piston down (figure 4a), then push the piston upward in order to remove any possible bubble (figure 4b). Pull the piston down to the graduation mark corresponding to the quantity in milliliters (ml) prescribed by your doctor (figure 4C).
|
ll |
1 |
1 |
l | ||
|
p |
} |
I |
k |
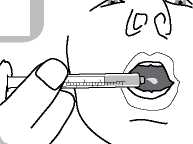
• Administer the contents of the syringe into the mouth by pushing the piston to the bottom of the syringe (figure 6) and ensure the medicine is swallowed.
Remove the adaptor from the bottle and close the bottle with the plastic screw cap.
Wash the adaptor and the syringe with warm water. Dry them with a clean paper towel and replace them into the box with your medicine.
If you take more Tapclob® Oral Suspension than you should
If you take more Tapclob® Oral Suspension than you should, tell your doctor or go to your nearest hospital casualty department immediately. Do not drive yourself because you may start to feel sleepy.
If you forget to take Tapclob® Oral Suspension If you have missed a dose take it as soon as you remember unless it is almost time for the next one then carry on as before. Do not take a double dose to make up for a forgotten dose.
If you stop taking Tapclob® Oral Suspension Do not stop taking your medicine without telling your doctor as he may gradually reduce your dose before stopping it completely. If stopped suddenly, you may have unpleasant side effects including stress (anxiety), confusion, or depression. You may also lose your appetite and have difficulty sleeping (see Section 2 'Dependence, tolerance and withdrawal').
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately if you have any of the following side effects. These effects are more likely to occur in children and the elderly:
• feeling restless, have difficulty sleeping or nightmares
• feeling irritable or anxious
• believing things which are not true (delusions)
• sensing things which are not there (hallucinations)
• feeling suicidal (see Section 2 'Suicidal thoughts')
The following side effects are more likely to occur at the start of treatment. They usually last for a short time:
• feeling sleepy or dizzy
• dry mouth, constipation
• loss of appetite, feeling sick
• shaking fingers
Other side-effects include:
• headache
• breathing problems
• loss of memory, confusion or inappropriate behaviour
• skin rash
• muscle weakness
• problems walking or other movement problems
• being aggressive
• reacting to things more slowly than usual
• problems with your vision such as double vision
• difficulty in staying awake or alert
• becoming dependent on Tapclob® Oral Suspension (also called 'physical or mental dependence') (see Section 2 'Dependence, tolerance and withdrawal')
• weight gain
• loss of sexual drive
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor, pharmacist or nurse.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme Website: http://www. mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Tapclob® Oral Suspension
Keep this medicine out of the sight and reach of children.
Do not use Tapclob® Oral Suspension after the expiry date stated on the bottle label and carton after EXP. The expiry date refers to the last day of that month.
Do not store above 25°C. Use within 28 days of opening. Keep the bottle in the outer carton.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6.Contents of the pack and other information
What Tapclob® Oral Suspension contains
• The active substance (the ingredient that makes the oral suspension work) is Clobazam.
Each 5ml of Tapclob® 5mg/5ml Oral Suspension contains 5mg of clobazam.
Each 5ml of Tapclob® 10mg/5ml Oral Suspension contains 10mg of clobazam.
• The other ingredients are sorbitol (E420), xanthan gum (E415), acesulfame potassium (E950), raspberry flavour, sodium propyl hydroxybenzoate (E217), sodium methyl hydroxybenzoate (E219), disodium hydrogen phosphate dihydrate, sodium dihydrogen phosphate dihydrate and purified water.
What Tapclob® Oral Suspension looks like and contents of the pack
Tapclob® Oral Suspension is an off white viscous suspension with an odour of raspberry supplied in an amber glass bottle.
The contents may settle during storage and should be shaken before use.
Pack sizes are 100ml, 150ml and 250ml.
Manufacturer and Marketing Authorisation Holder
Martindale Pharmaceuticals Ltd Trading as Martindale Pharma Bampton Road, Harold Hill, Romford, Essex,
RM3 8UG, United Kingdom
If you would like any more information, or would like the leaflet in a different format, please contact medical information at the above address.
Product licence numbers: PL 00156/0322 PL 00156/0323
This leaflet was last revised in July 2015

MARTINDALE PHARMA
Bampton Road. Harold Hill, Romford, RM3 8UG, UK
D0304400000
3044-H
PACKAGE LEAFLET: INFORMATION FOR THE USER
Clobazam
o
LrsJ
O
■P
Si
JCD
P
P
O
o
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again
• If you have any further questions, ask your doctor, nurse or pharmacist.
• This medicine has been prescribed for you.
Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet
1. What Tapclob® Oral Suspension is and what it is used for
2. What you need to know before you take Tapclob® Oral Suspension
3. How to take Tapclob® Oral Suspension
4. Possible side effects
5. How to store Tapclob® Oral Suspension
6. Contents of the pack and other information
1. What Tapclob® Oral Suspension is and what it is used for
Tapclob® Oral Suspension contains clobazam which belongs to a group of medicines called benzodiazepines.
Clobazam works by having a calming effect on the brain.
Tapclob® Oral Suspension is used to treat:
Severe anxiety over a short time
Epilepsy (fits) over a longer time (in combination with
other treatments)
Mental illness such as schizophrenia (in combination with other treatments)
2. What you need to know before you take Tapclob® Oral Suspension
Do not take Tapclob® Oral Suspension:
If you are allergic (hypersensitive) to clobazam, other benzodiazepine medicines or any of the other ingredients of this medicine (listed in section 6).
If you are in the first three months of pregnancy or think you might be pregnant (see 'Pregnancy and breastfeeding' for more information).
If you are breast-feeding.
If you have ever had problems with drugs or alcohol dependence in the past.
If you suffer from an illness that causes muscle weakness (called 'myasthenia gravis1).
If you have severe liver problems.
If you have breathing problems.
I f you stop breathing for short periods during sleep (called 'sleep apnoea syndrome').
Warnings and precautions
Talk to your doctor before taking Tapclob® Oral Suspension: If you have problems with controlling your movements (called 'spinal or cerebellar ataxia').
If you have depression, irrational fears and obsessions.
If you have delusions (believing things which are not true) or hallucinations (sensing things which are not there).
If your liver or kidneys do not work as well as they should.
■ If you have ever become dependent upon another drug or alcohol.
Suicidal thoughts
Some patients have experienced suicidal thoughts while taking medicines containing clobazam, particularly if they are already depressed. If you start experiencing thoughts of suicide or harm towards yourself please tell your doctor immediately.
Dependence, tolerance and withdrawal
t is possible for you to become dependent on Tapclob® Oral Suspension if you take it for long periods of time, particularly if you have a history of heavy alcohol or drug use. This means that you may feel that you need to continue treatment with Tapclob® Oral Suspension in order to feel well (known as psychological dependence). f you suddenly stop taking Tapclob® Oral Suspension you may experience worsening of the symptoms you were originally being treated for, as well as mood changes, anxiety, sleep disturbance or restlessness. This is known as withdrawal and can be avoided by slowly reducing your dose. If you are worried about dependence or withdrawal please talk to your doctor.
f you take Tapclob® Oral Suspension for long periods of time for treatment of epilepsy it is possible that you may become tolerant to it, meaning that it will not be as effective as it was when you first started taking it. If you feel that Tapclob® Oral Suspension is no longer helping to control your symptoms please talk to your doctor, they may suggest you take a short break from this medicine.
Other medicines and Tapclob® Oral Suspension Please tell your doctor, pharmacist or nurse if you
are taking, have recently taken or might use any other medicines, including medicines obtained without a prescription. Especially: medicines for epilepsy (such as phenytoin, carbamazepine or valproic acid) medicines for depression (such as MAOIs, SSRI's or tricyclic anti-depressants - such as trazodone) medicines for severe mental illness called 'neuroleptics' (such as chlorpromazine, haloperidol and clozapine) painkillers (such as medicines containing codeine, dihydrocodeine or morphine) sleeping tablets (such as zolpidem) tranquilisers (such as diazepam, temazepam or lorazepam)
muscle relaxants (such as baclofen) antihistamines that make you sleepy (such as chlorphenamine, promethazine or diphenhydramine) lithium - used for a mental illness called 'bipolar disorder (mood changes between a state of high excitability or exaggerated emotions and depression) cimetidine (used to treat ulcers and heartburn)
Anaesthetics
f you are going to have an anaesthetic, tell your doctor or anaesthetist you are taking Tapclob® Oral Suspension. This s because your doctor may need to change the amount of anaesthetic or muscle relaxants given to you.
Tapclob® Oral Suspension with food, drink and alcohol Do not drink alcohol while you are taking Tapclob® Oral Suspension. This is because alcohol can change the way Tapclob® Oral Suspension works.
Pregnancy and breast-feeding
Do not take Tapclob® Oral Suspension if you are in the first three months of pregnancy are breast-feeding
f you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor ,:or advice before taking this medicine. Tapclob® Oral Suspension should be avoided during pregnancy.
f you take Tapclob® Oral Suspension late in pregnancy or during labour your baby might have a low body temperature, floppiness, breathing or feeding difficulties. f taken regularly during late pregnancy, your baby may develop withdrawal symptoms.
Driving and using machines
You may feel sleepy or have concentration or memory problems after taking this medicine.
You may also experience double vision or you may react more slowly to things. Do not drive or use any tools or machines if you are affected in this way.
The medicine can affect your ability to drive as it may make you sleepy or dizzy.
Do not drive while taking this medicine until you know how it affects you.
It is an offence to drive if this medicine affects your ability to drive.
However, you would not be committing an offence if:
• The medicine has been prescribed to treat a medical or dental problem and
• You have taken it according to the instructions given by the prescriber or in the information provided with the medicine and
• It was not affecting your ability to drive safely
Talk to your doctor or pharmacist if you are not sure whether it is safe for you to drive while taking this medicine.
Tapclob® Oral Suspension contains:
Sorbitol (E420): If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product Sodium methyl hydroxybenzoate (E219) and sodium propyl hydroxybenzoate (E217): These may cause allergic reactions in some people that could occur some time after taking this medicine. The signs may include a rash, swallowing or breathing problems and swelling of your lips, face, throat or tongue
f you have any concerns over whether this medicine is suitable for you talk to your doctor, pharmacist or nurse.
3.How to take Tapclob® Oral Suspension
Always take this medicine exactly as your doctor has told you. Tapclob® Oral Suspension is usually given for 2 to 4 weeks. After that, your doctor will decide whether you should keep taking this medicine. Check with your doctor or pharmacist if you are not sure.
When you are taking Tapclob Oral Suspension you should not change to any different clobazam containing medicines except under your doctor's supervision.
5mg/5ml: Doses
Adults and adolescents (over 15 years)
The usual dose is 20mg (20ml) to 30mg (30ml) each day. This can be taken as two separate doses or as a single dose at night.
Your doctor may increase your dose to up to 60mg (60ml) each day.
Your doctor may lower the dose to suit you.
Children (over 6 years)
The usual starting dose is 5mg (5ml) each day.
Your doctor will then adjust the dose according to your child's need.
Elderly
The usual dose for anxiety is 10mg (10ml) to 20mg (20ml) each day.
10mg/5ml: Doses
Adults and adolescents (over 15 years)
The usual dose is 20mg (10ml) to 30mg (15ml) each day. This can be taken as two separate doses or as a single dose at night.
Your doctor may increase your dose to up to 60mg (30ml) each day.
Your doctor may lower the dose to suit you.
Children (over 6 years)
The usual starting dose is 5mg (2.5ml) each day.
Your doctor will then adjust the dose according to your child's need.
Elderly
The usual dose for anxiety is 10mg (5ml) to 20mg (10ml) each day.
Method of administration:
This product may settle during storage, please shake well before use.
Your doctor, pharmacist or nurse will show you how to administer this medicine. The box containing this medicine will contain a 5ml dosing syringe, a dosing adaptor and a 30ml dosing cup.
iiiiliiiiliinliiiiliiiil
5ml syringe each numbered increment is 1ml equivalent to 1mg of Tapclob 5mg/5ml Oral Suspension and 2mg of Tapclob 10mg/5ml Oral Suspension . The smaller increments are 0.2ml or 0.2mg of Tapclob 5mg/5ml Oral Suspension and 0.4mg of Tapclob 10mg/5ml Oral Suspension.

30ml dosing cup. Each numbered increment is 5ml -equivalent to 5mg of Tapclob 5mg/5ml Oral Suspension and 10mg of Tapclob 10mg/5ml Oral Suspension.
Instructions are provided overleaf for using the dosing syringe. If you have any questions about the dose you should use or how to use the syringe, you should ask your pharmacist.
Continued overleaf
3047-D
• Holding the bottle, take the plastic syringe adaptor from the box and insert the adaptor into the bottle neck (figure 2).
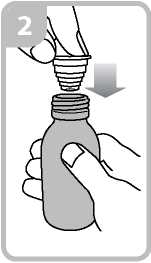
• Take the syringe and put it in the adaptor opening (figure 3).
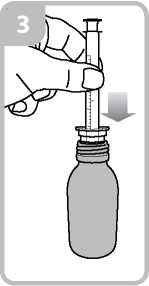
• Turn the bottle the right way up. Remove the syringe from the adaptor
(figure 5).
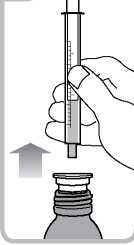
6
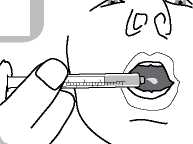
nstructions for use:
• Open the bottle: press the cap and turn it anticlockwise (figure 1)
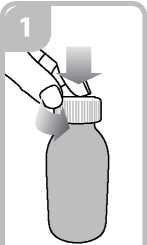
Vi
Ensure it is well fixed.
• Turn the bottle upside down.
• Fill the syringe with a small amount of suspension by pulling the piston down (figure 4a), then push the piston upward in order to remove any possible bubble (figure 4b). Pull the piston down to the graduation mark corresponding to the quantity in milliliters (ml) prescribed by your doctor (figure 4C).
|
ll |
1 |
1 |
l | ||
|
p |
} |
I |
k |
• Administer the contents of the syringe into the mouth by pushing the piston to the bottom of the syringe (figure 6) and ensure the medicine is swallowed.
Remove the adaptor from the bottle and close the bottle with the plastic screw cap.
Wash the adaptor and the syringe with warm water. Dry them with a clean paper towel and replace them into the box with your medicine.
If you take more Tapclob® Oral Suspension than you should
If you take more Tapclob® Oral Suspension than you should, tell your doctor or go to your nearest hospital casualty department immediately. Do not drive yourself because you may start to feel sleepy.
If you forget to take Tapclob® Oral Suspension If you have missed a dose take it as soon as you remember unless it is almost time for the next one then carry on as before. Do not take a double dose to make up for a forgotten dose.
If you stop taking Tapclob® Oral Suspension Do not stop taking your medicine without telling your doctor as he may gradually reduce your dose before stopping it completely. If stopped suddenly, you may have unpleasant side effects including stress (anxiety), confusion, or depression. You may also lose your appetite and have difficulty sleeping (see Section 2 'Dependence, tolerance and withdrawal').
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor immediately if you have any of the following side effects. These effects are more likely to occur in children and the elderly:
• feeling restless, have difficulty sleeping or nightmares
• feeling irritable or anxious
• believing things which are not true (delusions)
• sensing things which are not there (hallucinations)
• feeling suicidal (see Section 2 'Suicidal thoughts')
The following side effects are more likely to occur at the start of treatment. They usually last for a short time:
• feeling sleepy or dizzy
• dry mouth, constipation
• loss of appetite, feeling sick
• shaking fingers
Other side-effects include:
• headache
• breathing problems
• loss of memory, confusion or inappropriate behaviour
• skin rash
• muscle weakness
• problems walking or other movement problems
• being aggressive
• reacting to things more slowly than usual
• problems with your vision such as double vision
• difficulty in staying awake or alert
• becoming dependent on Tapclob® Oral Suspension (also called 'physical or mental dependence') (see Section 2 'Dependence, tolerance and withdrawal')
• weight gain
• loss of sexual drive
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor, pharmacist or nurse.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme Website: http://www. mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Tapclob® Oral Suspension
Keep this medicine out of the sight and reach of children.
Do not use Tapclob® Oral Suspension after the expiry date stated on the bottle label and carton after EXP. The expiry date refers to the last day of that month.
Do not store above 25°C. Use within 28 days of opening. Keep the bottle in the outer carton.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6.Contents of the pack and other information
What Tapclob® Oral Suspension contains
• The active substance (the ingredient that makes the oral suspension work) is Clobazam.
Each 5ml of Tapclob® 5mg/5ml Oral Suspension contains 5mg of clobazam.
Each 5ml of Tapclob® 10mg/5ml Oral Suspension contains 10mg of clobazam.
• The other ingredients are sorbitol (E420), xanthan gum (E415), acesulfame potassium (E950), raspberry flavour, sodium propyl hydroxybenzoate (E217), sodium methyl hydroxybenzoate (E219), disodium hydrogen phosphate dihydrate, sodium dihydrogen phosphate dihydrate and purified water.
What Tapclob® Oral Suspension looks like and contents of the pack
Tapclob® Oral Suspension is an off white viscous suspension with an odour of raspberry supplied in an amber glass bottle.
The contents may settle during storage and should be shaken before use.
Pack sizes are 100ml, 150ml and 250ml.
Marketing Authorisation Holder
Martindale Pharmaceuticals Ltd Trading as Martindale Pharma Bampton Road, Harold Hill, Romford, Essex,
RM3 8UG, United Kingdom Manufacturer
Nupharm Laboratories Limited 2 Newtech Square, Deeside Industrial Park,
Deeside, Flintshire, CH5 2NT United Kingdom
If you would like any more information, or would like the leaflet in a different format, please contact medical information at the above address.
Product licence numbers: PL 00156/0322 PL 00156/0323
This leaflet was last revised in July 2015

MARTINDALE PHARMA
Bampton Road, Harold Hill, Romford, RM3 8UG, UK
D0304700000
3047-D
100mm Measurement Verification Bar