Tarmed
GlaxoSmithKline Artwork Information Panel
RSCA/W
Version:
6
Polytar Scalp Shampoo
Coal tar solution 4%
Item Number: DEVCOMP-0003918
Manufacturing Site: *N/A
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
This medicine is available without prescription. However, you still need to read carefully to get the best results from it.
Keep this leaflet. You may need to read it again.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Polytar Scalp Shampoo is and what it is used for
2. What you need to know before you use Polytar Scalp Shampoo
3. How to use Polytar Scalp Shampoo
4. Possible side effects
5. How to store Polytar Scalp Shampoo
6. Content of this pack and other information
1 Ifl/hat Pnll/t^r Cfalrt ChamwftA ic and mihaf if ic ■ 1Cnrl fnv___
■ ■ im iiti L rvriy mi Jvwiim •jhi ia■ ■ ■ i/Wr is d■ ■ ve vui id i 11 is usc\« ■ wr
Polytar Scalp Shampoo is a shampoo containing the active ingredient coal tar. This is a thick, heavy oil which can reduce scales, inflammation and itchiness.
Polytar Scalp Shampoo is used to treat scalp conditions such as psoriasis (thickened patches of inflamed, red skin, often covered by silvery scales), seborrhoeic dermatitis (red, scaly, itchy scalp), eczema (itchy skin rash), itchiness and scaling due to these conditions and dandruff.
2. What you need to know before you use Polytar Scalp Shampoo
□
Do not use Polytar Scalp Shampoo:
If you have ever had an allergic reaction to coal tar, or any of the ingredients (listed in Section 6). On infected open or damaged skin, or pus-filled blisters (pustules).
If you are under 12 years.
Take care with this medicine
Market or Pack Owner:
CH-UK Regulatory-GBR; CH-United Kingdom-GBR
Market Trade Name: Polytar
Colour Standard Reference Number: N/A
Technical Reference No(s).: S1LFLT-2D
(do NOT include the technical referent® doc[s] version no[s].)
Printing Process: N/A
- Cai
Polytar Scalp Shampoo may cause irritancy if in contact with sensitive areas, such as broken or inflamed skin. Stop using Polytar Scalp Shampoo if irritation develops.
Avoid contact with the eyes. If it gets in your eyes, rinse with clean water.
Avoid exposure to strong sunlight or sun beds. If exposure cannot be avoided, wear protective clothing and sunscreen.
Can stain skin or clothes and can rarely cause a temporary change in hair colour.
O)
c
If you are taking other medicines:
Talk to your doctor or pharmacist before using this medicine if you are taking any prescribed medicines particularly ones that may make you more sensitive to sunlight such as thiazides (water tablets), some antibiotics (e.g. tetracyclines) and tranquilizers (e.g. phenothiazines).
Pregnancy and breast-feeding
Talk to your doctor before using this product if you are pregnant or breast feeding. Do not use this medicine if you are in the first trimester of pregnancy. If used during breast feeding (after consultation with your doctor), make sure all coal tar is washed off the breast before feeding.
DEVCOMP-
0003918
Artwork copyright is the property of the GlaxoSmithKline Group of Companies
All suppliers providing a service to GSK for printed components of any description must ensure that they have a licence for all fonts/software used in conjunction with GSK artwork.
The distribution and use of fonts / software without a licence constitutes an intellectual property infringement. G5K will not accept any liability for the breach of third party intellectual property rights by printed component suppliers.
The GSK certification / audit process requires suppliers to declare that they do not use unlicensed fonts / software and may require the supplier to produce evidence of such licence to GSK.
s
'5
c
CD
T3
ra
ATTENTION • ATTENTION
To Ensure Accurate PDF Viewing and Printing: FOR SCREEN VIEWING: Use Adobe Acrobat 7 Professional or Adobe Acrobat Reader, Standard or Professional (higher than 7). Overprint Preview must be activated for accurate on screen viewing. FOR PRINTING: Use only Acrobat Professional version 7 or higher. "Apply Overprint Preview” or "Simulate Overprinting" must be activated in the print settings for printing accurate hard copies.
GSK Market is responsible for this product, its design and content.
Ensure the artwork is thoroughly checked, all the text proof-read and approved.
RSC GSK is responsible for site technical requirements and pre-press suitability.
TEXT SIZE CONTAINED IN THIS ARTWORK
Body text size: 7.5pt Leading: 7.6pt Horizontal Scale: 100% Smallest text size: 7.5pt Microtext: Y
GSK Market
is responsible to advise RSC in case changes required impact the followings:
Formulation Tablet embossing Storage conditions
Cholf I ifo
Always use this medicine exactly as described in this leaflet or as your doctor or pharmacist has told you. Check with your doctor or pharmacist, if you are not sure.
Adults and children aged 12 years and over:
Use Polytar Scalp Shampoo once or twice a week for four weeks. Use for longer than this should only be on the advice of a doctor.
1. Wet your hair.
2. Use enough shampoo so that the lather is abundant to cover your scalp, hair and adjacent areas, if also affected.
3. Massage the scalp and adjacent areas well using your fingertips.
4. Rinse your scalp and hair thoroughly.
5. Repeat steps 2 to 4.

Polytar Scalp Shampoo should be in contact with your scalp for a total time of three to five minutes over the two applications.
If you use more Polytar Scalp Shampoo than you should
If you use more Polytar Scalp Shampoo than recommended or use more often than recommended, you may be more likely to get skin irritation or skin sensitivity to sunlight.
If you forget to use Polytar Scalp Shampoo
If you forget to use Polytar Scalp Shampoo just apply the next day instead.
4. Possible side effects
Like all medicines, Polytar Scalp Shampoo can cause side effects, although not everybody gets them. A small number of people have had side effects.
Stop taking this medicine and tell your doctor immediately if you experience:
- Allergic reactions. These may include developing a rash, swelling of the mouth or face or having difficulty breathing.
Stop taking the medicine and tell your doctor if you experience:
• eye irritation
• unusual hair loss or thinning
• eczema (itchy skin rash)
• hair colour changes
• abnormal hair texture
• sensitivity to sunlight
• skin irritation, skin may be dry, red or itchy, you may feel a burning sensation on the skin
• skin pain, rash or swelling at the application site
If any of the side effects get serious, last longer than a few days or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist. Also you can help make sure that medicines remain as safe as possible by reporting any unwanted side effects via the internet at www.mhra.gov.uk/yellowcard; alternatively you can call Freephone 0808 100 3352 (available between 10am-2pm Monday-Friday) or fill in a paper form available from your local pharmacy.
Store below 25°C.
Do not use Polytar Scalp Shampoo after the expiry date on the bottle and the carton. The expiry date refers to the last day of that month.
6. Further Information
Active ingredient: Coal tar solution 4% (w/w).
Other ingredients: Sodium Lauryl Ether Sulfate, Cocamidopropyl Betaine, Disodium Phosphate Dihydrate, Citric Acid Monohydrate, Benzyl Alcohol, Macrogol 150 Distearate, Hexylene Glycol, Oleyl Alcohol, Polyquaternium 10, Fruitier Timotei Fragrance, Purified Water.
What Polytar Scalp Shampoo looks like and contents of the pack Bottles contain 25ml, 150ml and 250ml. Not all pack sizes may be marketed.
The marketing authorisation holder is Stiefel, 980 Great West Road, Brentford, Middlesex,
TW8 9GS, U.K. and all enquiries should be sent to this address.
Manufactured by:
Stiefel Laboratories (Ireland) Ltd., Finisklin Business Park, Sligo, Ireland.
This leaflet was last revised: July 2015.
Polytar is a registered trade mark used under license by Stiefel Laboratories, Inc.
DEVCOMP-
0003918
|
GlaxoSmithKline Artwork Information Panel |
RSC A/W Version: 6 | |||
|
Item Number: DEVCOMP-0003918 | ||||
|
Manufacturing Site: *N/A | ||||
|
Market or Pack Owner: CH-UK Regulatory-GBR; CH-United Kingdom-GBR | ||||
|
Market Trade Name: Polytar | ||||
|
Colour Standard Reference Number: N/A | ||||
|
Technical Reference No(s).: S1LFLT-2D (do NOT include the technical referent® doc[s] version no[s].) | ||||
|
Printing Process: N/A | ||||
|
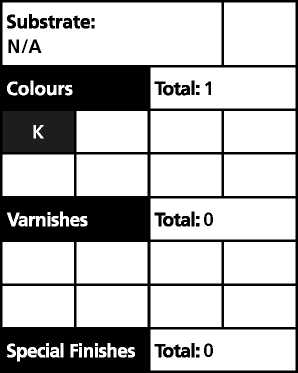
Substrate: N/A | ||||
|
Colours |
Total: 1 | |||
|
K | ||||
|
Varnishes |
Total: 0 | |||
|
Special Finishes |
Total: 0 | |||
|
Artwork copyright is the property of the GlaxoSmithKline Group of Companies All suppliers providing a service to GSK for printed components of any description must ensure that they have a licence for all fonts/software used in conjunction with GSK artwork. The distribution and use of fonts / software without a licence constitutes an intellectual property infringement. G5K will not accept any liability for the breach of third party intellectual property rights by printed component suppliers. The GSK certification / audit process requires suppliers to declare that they do not use unlicensed fonts / software and may require the supplier to produce evidence of such licence to GSK. | ||||
O)
c
S
c
o
B
To Ensure Accurate PDF Viewing and Printing: FOR SCREEN VIEWING: Use Adobe Acrobat 7 Professional or Adobe Acrobat Reader, Standard or Professional (higher than 7). Overprint Preview must be activated for accurate on screen viewing. FOR PRINTING: Use only Acrobat Professional version 7 or higher. "Apply Overprint Preview” or "Simulate Overprinting" must be activated in the print settings for printing accurate hard copies.
ATTENTION • ATTENTION
GSK Market is responsible for this product, its design and content.
Ensure the artwork is thoroughly checked, all the text proof-read and approved.
RSC GSK is responsible for site technical requirements and pre-press suitability.
TEXT SIZE CONTAINED IN THIS ARTWORK
Body text size: 7.5pt Leading: 7.6pt Horizontal Scale: 100% Smallest text size: 7.5pt Microtext: Y
GSK Market
is responsible to advise RSC in case changes required impact the followings:
Formulation Tablet embossing Storage conditions
Cholf I ifo
Page 2 of 2
This PDF has been verified using PitStop 12
PDF is PDF/X-4 compliant and GSK compliant for Leaflet workflow