Teglutik 5 Mg/Ml Oral Suspension
• anaemia
• allergic reactions
• inflammation of the pancreas (pancreatitis).
As riluzole oral suspension is more rapidly absorbed than riluzole tablets, a slight increase in tiredness, dizziness, diarrhoea and transaminases cannot be excluded. Reporting of side effects If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via Yellow Card Scheme at Website: www.mhra.gov.uk/ yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store TEGLUTIK
Keep out of the sight and reach of children.
Do not use TEGLUTIK after the expiry date which is stated on the carton and the bottle, after EXP. The expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
Once opened, use within 15 days.
Do not throw away any medicines via wastewater or household waste.
Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What TEGLUTIK contains
• The active substance is riluzole.
211922A/03
1 ml of oral suspension contains 5 mg of riluzole.
• The other ingredients are: Liquid Sorbitol (E420), Aluminum magnesium Silicate, Xanthan Gum, Saccharin Sodium, Simethicone emulsion 30%, Sodium Laurilsulphate, Macrogol Cetostearyl Ether, Purified Water.
What TEGLUTIK looks like and content of the pack
This medicine is presented as a slightly brown, opaque homogeneous oral suspension after being manually gently shaken. TEGLUTIK is available in a bottle of 250 and 300 ml with a plastic graduated oral dosing syringe. Pack sizes are:
- carton box containing one or two bottle of 250 ml of Riluzole 5 mg/ ml Oral Suspension.
- Carton box containing one bottle of 300 ml of Riluzole 5 mg/ml Oral Suspension.
The syringe barrel is graduated in milliliters up to 10 ml.
Marketing Authorisation Holder
ITALFARMACO S.A.
San Rafael, 3
28108 Alcobendas (Madrid) Spain Manufacturer ITALFARMACO S.A.
San Rafael, 3
28108 Alcobendas (Madrid) Spain
This leaflet was last revised in
07/2016
PACKAGE LEAFLET: INFORMATION FOR THE USER
TEGLUTIK 5 mg/ml oral suspension
Riluzole
D03050
Read all of this leaflet carefully before you start taking this medicine
because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What TEGLUTIK is and what it is used for
2. What you need to know before you take TEGLUTIK
3. How to take TEGLUTIK
4. Possible side effects
5. How to store TEGLUTIK
6. Contents of the pack and other information
1. What TEGLUTIK is and what it is used for
What TEGLUTIK is
The active substance in TEGLUTIK is riluzole which acts on the nervous system.
What TEGLUTIK is used for
TEGLUTIK is used in patients with amyotrophic lateral sclerosis (ALS). ALS is a form of motor neurone disease where attacks of the nerve cells responsible for sending instructions to the muscles lead to weakness, muscle waste and paralysis.
The destruction of nerve cells in motor neurone disease may be caused by too much glutamate (a chemical messenger) in the brain and spinal cord. TEGLUTIK stops the release of glutamate and this may help in preventing the nerve cells
being damaged.
Please consult your doctor for more information about ALS and the reason why this medicine has been prescribed for you.
2. What you need to know before you take TEGLUTIK Do not take TEGLUTIK
- if you are allergic to riluzole or any of the other ingredients of this medicine (listed in section 6),
- if you have any liver disease or increased blood levels of some enzymes of the liver
(transaminases),
- if you are pregnant or breastfeeding.
Warnings and precautions
Talk to your doctor or pharmacist before taking TEGLUTIK:
- if you have any liver problems:
yellowing of your skin or the white of your eyes (jaundice), itching all over, feeling sick, being sick;
- if your kidneys are not working very well;
- if you have any fever: it may be due to a low number of white blood cells which can cause an increased risk of infection;
Continued overleaf
Codigo de Barras Sentido de Lectura Barcode Read Direction
UlilT
81
A
If any of the above applies to you, or if you are not sure, tell your doctor who will decide what to do.
Children and Adolescents
If you are less than 18 years of age, the use of TEGLUTIK is not recommended in children because there is no information available in this population.
Other medicines and TEGLUTIK
Tell your doctor if you are taking or have recently taken or might take any other medicines, including medicines obtained without a prescription. Pregnancy, breast-feeding and fertility
You must not take TEGLUTIK if you are pregnant, think you may be pregnant, or if you are breast feeding. If you think you may pregnant or if you intend to breast-feed, ask your doctor for advice before taking this medicine.
Driving and using machines
You can drive or use any tools or machines, unless you feel dizzy or light headed after taking this medicine. TEGLUTIK contains liquid sorbitol (E420).
If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
3. How to take TEGLUTIK
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is 100 mg a day (50 mg every 12 hours).
10 ml of the oral suspension, containing 50 mg of riluzole, should be taken by mouth every 12 hours, at the same time of the day each
day (for example, in the morning and evening). The suspension is administered by means of graduated dosing syringe.
The oral suspension must be manually gently shaken for at least 30 seconds by rotating the bottle by 180° and the homogeneity should be visually verified.
Method of administration:
Instructions for use:
Open the bottle: press the cap and turn it anticlockwise (figure 1)
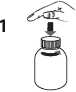
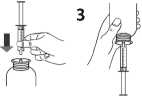
Take the syringe, remove the tip and insert the syringe in the adaptor opening (figure 2). Turn the bottle upside down (figure 3).
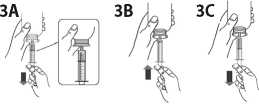
Fill the syringe with a small amount of suspension by pulling the plunger down (figure 3A), then push the piston upward in order to remove any possible bubble (figure 3B). Pull the piston down to the graduation mark corresponding to the quantity in milliliters (ml) prescribed by your doctor (figure 3C).
Turn the bottle the right way up (figure 4A). Remove the syringe from the adaptor (figure 4B).
4A 4B
• Administer orally the whole content of the syringe. Dilution in water is not necessary.
• Close the bottle with the plastic screw cap.
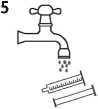
• Wash the syringe with water only and re-assemble it with its tip cap once dried (figure 5).
If you take more TEGLUTIK than you should
If you take too much suspension, contact your doctor or the nearest hospital emergency department immediately.
If you forget to take TEGLUTIK
If you forget to take your dose, leave out that dose completely and take the next dose at the usual time.
Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects Like all medicines, TEGLUTIK can cause side effects, although not everybody gets them.
Important
Tell your doctor immediately
• if you experience any fever (increase in temperature) because TEGLUTIK may cause a decrease in the number of white blood cells. Your doctor may want to take a blood sample to check the number of white blood cells, which are important in fighting infections.
• if you experience any of the following symptoms: yellowing of your skin or the white of your eyes (jaundice), itching all over, feeling sick, being sick, as these may be signs of liver disease (hepatitis). Your doctor may do regular blood tests while you are taking TEGLUTIK to make sure that this does not occur.
• if you experience cough or difficulties in breathing, as this may be a sign of lung disease (called interstitial lung disease).
Other side effects
Very common side effects
(may affect more than 1 in 10 people)
• tiredness
• feeling sick
• increased blood levels of some enzymes of the liver (transaminases).
Common side effects
(may affect up to 1 in 10 people):
• dizziness
• numbness or tingling of the mouth
• vomiting
• sleepiness
• increase in heart beat
• diarrhoea
• headache
• abdominal pain
• pain
Uncommon side effects
(may affect up to 1 in 100 people)