Tekcis 2-50 Gbq Radionuclide Generator
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Tekcis 2-50 GBq radionuclide generator
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Sodium pertechnetate (99mTc) injection is produced by means of a (99Mo/99mTc) radionuclide generator. Technetium (99mTc) decays with the emission of gamma radiation with a mean energy of 140 keV and a half-life of 6.02 hours to technetium (99Tc) which, in view of its long half-life of 2.13 x 105 years, can be regarded as quasi stable.
The radionuclide generator containing the parent isotope 99Mo, adsorbed to a chromatographic column, delivers sodium pertechnetate (99mTc) injection in sterile solution.
The 99Mo on the column is in equilibrium with the formed daughter isotope 99mTc. The generators are supplied with the following 99Mo activity amounts at activity reference time which delivers in equilibrium the following technetium (99mTc) amounts :
|
99mTc activity (Maximal eluable activity at calibration date, 12h CET) |
2 |
4 |
6 |
8 |
10 |
12 |
1 6 |
20 |
25 |
50 |
GBq |
|
99Mo activity |
2.5 |
5 |
7 |
9.5 |
12 |
14. |
1 |
24 |
30 |
60 |
GBq |
|
(at calibration date, |
5 |
9 | |||||||||
|
12h CET) |
Excipient with known effect: sodium
Each mL of sodium pertechnetate(99mTc) solution contains 3.6 mg of sodium. For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Radionuclide generator
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
This medicinal product is for diagnostic use only.
The eluate from the radionuclide generator (Sodium Pertechnetate (99mTc)
Injection Ph. Eur.) is indicated for:
• Labelling of various kits for radiopharmaceutical preparation developed and approved for radiolabelling with such solution.
• Thyroid scintigraphy: direct imaging and measurement of thyroid uptake to give information on the size, position, nodularity and function of the gland in thyroid disease.
• Salivary gland scintigraphy: diagnosis of chronic sialadenitis (e.g. Sjogren Syndrom) as well as to assess salivary gland function and duct patency in salivary glands disorders and monitoring of the response to therapeutic interventions (in particular radio iodine therapy).
• Location of ectopic gastric mucosa (Meckel's diverticulum).
Lacrimal duct scintigraphy to assess functional disorders of lacrimation and monitoring of the response to therapeutic interventions.
4.2 Posology and method of administration
This medicinal product is for use in designated nuclear medicine facilities only, and should only be handled by authorised personnel.
Posology
Sodium pertechnetate (99mTc) is normally administered intravenously at activities which vary widely according to the clinical information required and the equipment employed. Other activities may be justifiable. The injection of activities greater than local DRLs (Diagnostic Reference Levels) should be justified. Pre-treatment of patients with thyroid blocking agents or reducing agents may be necessary for certain indications. Recommended activities are as follows:
Adults (70 kg) and elderly population:
• Thyroid scintigraphy: 20-80 MBq
• Salivary gland scintigraphy: 30 to 150 MBq for static images up to 370 MBq for dynamic images.
• Meckel's diverticulum scintigraphy: 300-400 MBq
• Lacrimal duct scintigraphy: 2-4 MBq each eye.
Renal impairment
Careful consideration of the activity to be administered is required since an increased radiation exposure is possible in these patients.
Paediatric population
The use in children and adolescents has to be considered carefully, based upon clinical needs and assessing the risk/benefit ratio in this patient group.
The activities to be administered to children and to adolescents may be calculated according to the European Association of Nuclear Medicine (EANM-May 2008) guidelines, by using the formula corresponding to the indication concerned and the relevant correction factor corresponding to the body mass of the young patient (see Table 1).
Thyroid scintigraphy:
Activity administered [MBq] = 5.6 MBq x Correction factor (Table 1),
With a minimal activity of 10 MBq necessary for obtaining quality images satisfactory.
Identification of ectopic gastric mucosa:
Activity administered [MBq] = 10.5 MBq x Correction factor (Table 1), Minimal activity: 20 MBq.
Table 1
|
Mass |
factor |
Mass |
factor |
Mass |
factor |
|
3 kg |
= 1 |
22 kg |
= 5.29 |
42 kg |
= 9.14 |
|
4 kg |
= 1.14 |
24 kg |
= 5.71 |
44 kg |
= 9.57 |
|
6 kg |
= 1.71 |
26 kg |
= 6.14 |
46 kg |
= 10.00 |
|
8 kg |
= 2.14 |
28 kg |
= 6.43 |
48 kg |
= 10.29 |
|
10 kg |
= 2.71 |
30 kg |
= 6.86 |
50 kg |
= 10.71 |
|
12 kg |
= 3.14 |
32 kg |
= 7.29 |
52-54 kg |
= 11.29 |
|
14 kg |
= 3.57 |
34 kg |
= 7.72 |
56-58 kg |
= 12.00 |
|
16 kg |
= 4.00 |
36 kg |
= 8.00 |
60-62 kg |
= 12.71 |
|
18 kg |
= 4.43 |
38 kg |
= 8.43 |
64-66 kg |
= 13.43 |
|
20 kg |
= 4.86 |
40 kg |
= 8.86 |
68 kg |
= 14.00 |
Salivary gland scintigraphy:
The Paediatric Task Group of EANM (1990) recommends that the activity to be administered to a child should be calculated from the body weight according to the following table 2:
Table 2 : Fraction of adult activity :
|
3 kg |
= 0.1 |
22 kg |
= 0.50 |
42 kg |
= 0.78 |
|
4 kg |
= 0.14 |
24 kg |
= 0.53 |
44 kg |
= 0.80 |
|
6 kg |
= 0.19 |
26 kg |
= 0.56 |
46 kg |
= 0.82 |
|
8 kg |
= 0.23 |
28 kg |
= 0.58 |
48 kg |
= 0.85 |
|
10 kg |
= 0.27 |
30 kg |
= 0.62 |
50 kg |
= 0.88 |
|
12 kg |
= 0.32 |
32 kg |
= 0.65 |
52-54 kg |
= 0.90 |
|
14 kg |
= 0.36 |
34 kg |
= 0.68 |
56-58 kg |
= 0.92 |
|
16 kg |
= 0.40 |
36 kg |
= 0.71 |
60-62 kg |
= 0.96 |
18 kg = 0.44 38 kg = 0.73 64-66 kg = 0.98
20 kg = 0.46 40 kg = 0.76 68 kg = 0.99
In very young children a minimum dose of 10 MBq is necessary in order to obtain images of sufficient quality.
Lacrimal duct scintigraphy : recommended activities apply as well for adults as for children.
Method of administration For multidose use.
For intravenous or ocular use or labeling.
For instructions on extemporary preparation of the medicinal product before administration, see section 12.
For patient preparation, see section 4.4.
In thyroid scintigraphy, salivary gland scintigraphy and identification of ectopic gastric mucosa, the sodium pertechnetate (99mTc) solution is administered by intravenous injection.
In lacrimal duct scintigraphy, drops are instilled in each eye.
Image acquisition
Thyroid scintigraphy: 20 minutes after intravenous injection.
Salivary gland scintigraphy: Static images performed immediately after intravenous injection and at regular intervals up to 15 minutes.
Dynamic images performed immediately after injection and at regular intervals up to 30 minutes.
The dynamic acquisition is recommended.
Identification of ectopic gastric mucosa: immediately after intravenous injection and at regular intervals for 30 minutes.
Lacrimal duct scintigraphy: dynamic acquisition within 2 minutes after instillation, followed by static images acquired at regular intervals within 20 minutes.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
4.4 Special warnings and precautions for use
Potential for hypersensitivity or anaphylactic reactions
If hypersensitivity or anaphylactic reactions occur, the administration of the medicinal product must be discontinued immediately and intravenous treatment initiated, if necessary. To enable immediate action in emergencies, the necessary medicinal products and equipment such as endotracheal tube and ventilator must be immediately available.
Individual benefit/risk justification
For each patient, the radiation exposure must be justifiable by the likely benefit. The activity administered should in every case be as low as reasonably achievable to obtain the required diagnostic information.
Renal impairment
Careful consideration of the benefit risk ratio in these patients is required since an increased radiation exposure is possible.
Paediatric population
For information on the use in paediatric population, see section 4.2.
Careful consideration of the indication is required since the effective dose per MBq is higher than in adults (see section 11).
Patient preparation
Pretreatment of patients with thyroid-blocking agents or reducing agents may be necessary for certain indications.
The patient should be well hydrated before the start of the examination and urged to void as often as possible during the first hours after the examination in order to reduce radiation.
In Meckel’s diverticulum scintigraphy, the patient should be fasting for 3 to 4 hours prior to examination, to keep the small bowel peristalsis low.
After the procedure
Close contact with infants and pregnant women should be restricted during 12h. Specific warnings
Sodium pertechnetate (99mTc) solution for injection contains 3.6 mg/mL of sodium.
Depending on the time when you administer the injection, the content of sodium given to the patient may in some cases be greater than 1 mmol. This should be taken into account in patient on low sodium diet.
When labelling kit, the sodium content of the dose administered must take into account the sodium derived from the eluate and the kit. Please refer to the package leaflet of the kit considered.
To avoid false positives or to minimize irradiation by reduction of pertechnetate accumulation in the thyroid and salivary glands, potassium perchlorate should be given prior to lacrimal duct scintigraphy or Meckel diverticulum scintigraphy.
In salivary gland scintigraphy a lower specificity of the method should be expected compared to MR sialography.
Precautions with respect to environmental hazard see section 6.6.
4.5 Interaction with other medicinal products and other forms of interaction
Atropine, isoprenaline and analgesics may cause a delay of gastric emptying and thereby cause a redistribution of (99mTc) pertechnetate in abdominal imaging.
Administration of laxatives should be withheld since they irritate the gastrointestinal tract. Contrast-enhanced studies (e.g. barium) and upper GI examination should be avoided within 48 h prior to administration of pertechnetate (99mTc) for Meckel’s diverticulum .scintigraphy.
Many pharmacological agents are known to modify the thyroid uptake.
- antithyroid agents (e.g. carbimazole or other imidazole derivatives such as propylthiouracil), salicylates, steroids, sodium nitroprusside, sodium sulfobromophtalein, perchlorate should be withheld for 1 week prior thyroid scintigraphy ;
- phenylbutazone and expectorants should be withheld for 2 weeks ;
- natural or synthetic thyroid preparations (e.g. sodium thyroxine, sodium liothyronine, thyroid extract) should be withheld for 2-3 weeks ;
- amiodarone, benzodiazepines, lithium should be withheld for 4 weeks ;
- intravenous contrast agents should not have been administered within 1-2 months.
4.6 Fertility, Pregnancy and lactation
Women of childbearing potential
When an administration of radiopharmaceuticals to a woman of childbearing potential is intended, it is important to determine whether or not she is pregnant. Any woman who has missed a period should be assumed to be pregnant until proven otherwise. If in doubt about her potential pregnancy (if the woman has missed a period, if the period is very irregular, etc.), alternative techniques not using ionising radiation (if there are any) should be offered to the patient.
Pregnancy
99mTc (as free pertechnetate) has been shown to cross the placental barrier.
Radionuclide procedures carried out in pregnant women also involve radiation doses to the foetus. Only essential investigations should therefore be carried out during pregnancy, when the likely benefit far exceeds the risk incurred by the mother and foetus.
Direct administration of 400 MBq sodium pertechnetate (99mTc) to a patient results in an absorbed dose to the uterus of 3.2 mGy. Following pretreatment of patients with a blocking agent, administration of 400 MBq sodium pertechnetate (99mTc) results in an absorbed dose to the uterus of 2.4 mGy.
Breast-feeding
Before administering radiopharmaceuticals to a mother who is breast-feeding, consideration should be given to the possibility of delaying the administration of radionuclide until the mother has ceased breast-feeding, and to what is the most appropriate choice of radiopharmaceuticals, bearing in mind the secretion of activity in breast milk. If the administration is considered necessary, breast-feeding should be interrupted for 12 hours post administration and the expressed feeds discarded.
Close contact with infants should be restricted during this period.
4.7 Effects on ability to drive and use machines
The sodium pertechnetate (99mTc) solution has no or negligible influence on the ability to drive and use machines.
4.8. Undesirable effects
Information on adverse reactions is available from spontaneous reporting. The reported reaction types are anaphylactoid reactions, vegetative reactions, as well as different kinds of injection site reactions. Sodium perchtechnetate (99mTc) from the Tekcis generator is used for radioactive labelling of a variety of compounds. These pharmaceuticals generally have a higher potential for side effects than 99mTc, and therefore the reported side effects are rather related to the labelled compounds than to 99mTc. The possible types of side effects following intravenous administration of a 99mTc-labelled pharmaceutical preparation will be dependent on the specific compound being used. Such information can be found in the SmPC of the Kit used for radiopharmaceutical preparation.
Anaphylactoid reactions:
Anaphylactoid reactions have been reported following intravenous injection of perchtechnetate (99mTc) and include various skin or respiratory symptoms like skin irritations, oedema, or dyspnoea2.
Vegetative reactions (nervous system and gastrointestinal disorders):
Single cases of severe vegetative reactions have been reported, however, most of the reported vegetative effects include gastrointestinal reactions like nausea or vomiting2. Other reports include vasovagal reactions like headache2 or dizziness2. Vegetative effects are rather considered to be related to the examinational setting than to technetium (99mTc), especially in anxious patients.
General disorders and administration site conditions
Other reports describe local injection site reactions. Such reactions are related to extravasation of the radioactive material during the injection, and the reported reactions rank from local swelling2 up to cellulitis2. Depending on the administered radioactivity and the labeled compound, extended extravasation may necessitate surgical treatment.
The following table subsumes the observed reaction types and symptoms. Due to the fact that only spontaneous reports could be analysed, no frequency indications could be provided.
Adverse Reactions sorted by System Organ Class
Immune system disorders
Frequency unknown*: Anaphylactoid reactions (e.g. dyspnoea2, coma, urticaria, erythema, rash, pruritus, oedema at various location e.g. face oedema)
Nervous system disorders
Frequency unknown*: Vasovagal reactions (e.g. syncope, tachycardia, bradycardia, dizziness2, headache2, vision blurred, flushing)
Gastrointestinal disorders
Frequency unknown*: Vomiting2, nausea, diarrhoea2
General disorders and administration site conditions
Frequency unknown*: Injection site reactions (e.g. cellulitis2, pain2, erythema2, swelling2)
* Adverse reactions derived from spontaneous reporting
2 These adverse reactions have been reported with similar products and are therefore likely to occur with Tekcis.
Exposure to ionising radiation is linked with cancer induction and a potential for development of hereditary defects. As the effective dose is 5.2 mSv when the maximal recommended activity of 400 MBq is administered these adverse reactions are expected to occur with a low probability.
4.9 Overdose
In the event of administration of a radiation overdose with sodium pertechnetate (99mTc), the absorbed dose to the patient should be reduced where possible by increasing the elimination of the radionuclide from the body by forced diuresis and frequent bladder voiding and defecation.
The uptake in the thyroid, salivary glands and the gastric mucosa can be significantly reduced when sodium perchlorate is given immediately after an accidentally high dose of sodium pertechnetate (99mTc) was administered.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Diagnostic radiopharmaceuticals, ATC code: V09 No pharmacological activity has been observed in the range of doses administered for diagnostic purposes.
5.2 Pharmacokinetic properties
Distribution
The pertechnetate ion has similar biological distribution to iodide and perchlorate ions, concentrating.temporarily in salivary glands, choroid plexus, stomach (gastric mucosa) and in the thyroid gland, from which it is eliminated, unchanged. The pertechnetate ion also tends to concentrate in areas with increased vascularisation or with abnormal vascular permeability, particularly when pre-treatment with blocking agents inhibits uptake in glandular structures. With intact blood brain barrier pertechnetate (99mTc) does not penetrate into the brain tissue.
Organ uptake
In the blood 70-80% of the intravenously injected pertechnetate (99mTc) is bound to proteins, primarily in an unspecific way to albumin. The unbound fraction (20-30%) accumulates temporarily in thyroid and salivary glands, stomach and nasal mucous membranes as well as in the plexus chorioideus.
Pertechnetate (99mTc) in contrast to iodine, nevertheless, is neither used for the thyreoidal hormone synthesis (organification), nor absorbed in the small intestine. In the thyroid the maximum accumulation, depending on functional state and iodine saturation (in euthyreosis approx. 0.3-3%, in hyperthyreosis and iodine depletion up to 25%) is reached about 20 min after injection and then decreases quickly again. This also applies for the stomach mucous membrane parietal cells and the salivary glands acinar cells.
In contrast to the thyroid which releases pertechnetate (99mTc) again in the bloodstream it is secreted in the saliva and gastric juice. The accumulation by the salivary gland lies in the magnitude of 0.5% of the applied activity with the maximum reached after about 20 minutes. One hour after injection the concentration in the saliva is about 10-30 fold higher than in the plasma. The excretion can be accelerated by lemon juice or by stimulation of the parasympathetic nerve system, the absorption is reduced by perchlorate.
Elimination
Plasma clearance has a half-life of approximately 3 hours. pertechnetate (99mTc) is not metabolised in the organism. One fraction is eliminated very quickly renally, the rest more slowly via faeces, salivary and tear liquid. Excretion during the first 24 hours following administration is mainly urinary (approximately 25%) with faecal excretion occurring over the next 48 hours. Approximately 50% of the administered activity is excreted within the first 50 hours. When selective uptake of pertechnetate (99mTc) in glandular structures is inhibited by the preadministration of blocking agents, excretion follows the same pathways but there is a higher rate of renal clearance.
When sodium pertechnetate (99mTc) solution for injection is used for the production of technetium-99m-complexes pharmacologic as well as the toxicological qualities may change, depending on the kind of the respective technetium ligands.
5.3 Preclinical safety data
There is no information on acute, subacute and chronic toxicity from single or repeated dose administration. The quantity of sodium pertechnetate (99mTc) administered during clinical diagnostic procedures is very small and apart from allergic reactions, no other adverse reactions have been reported.
This medicinal product is not intended for regular or continous administration.
Mutagenicity srudies and long term carcinogenicity studies have not been carried out.
Reproductive Toxicity: Placental transfer of 99mTc from intravenously administered sodium pertechnetate (99mTc) has been studied in mice. The pregnant uterus was found to contain as much as 60% of the injected 99mTc when administered without perchlorate pre-administration. Studies performed on pregnant mice during gestation, gestation and lactation, and lactation alone showed changes in progeny which included weight reduction, hairlessness and sterility.
6. PHARMACEUTICAL PARTICULARS
6.1. List of excipients
Column system:
Aluminiumoxide.
Bag of solution for elution:
Sodium chloride, sodium nitrate, water for injection.
Elution vials:
Nitrogen under reduced pressure.
6.2 Incompatibilities
This medicinal product must not be mixed with other medicinal products except for those indicated in section 12.
6.3 Shelf life
Generator: 21 days from manufacturing date.
The calibration date and the expiry date are stated on the label.
Sodium pertechnetate (99mTc) eluate: After elution, use within 10 hours up to 10 withdrawals.
This medicinal product does not require any special storage conditions.
Elution vials: 24 months.
6.4 Special precautions for storage
Generator: This medicinal product does not require any special storage conditions.
Eluate: For storage conditions after elution of the medicinal product, see section 6.3. Vacuum vials: Do not store above 25°C.
Storage of radiopharmaceuticals should be in accordance with national regulation on radioactive materials.
6.5 Nature and contents of container
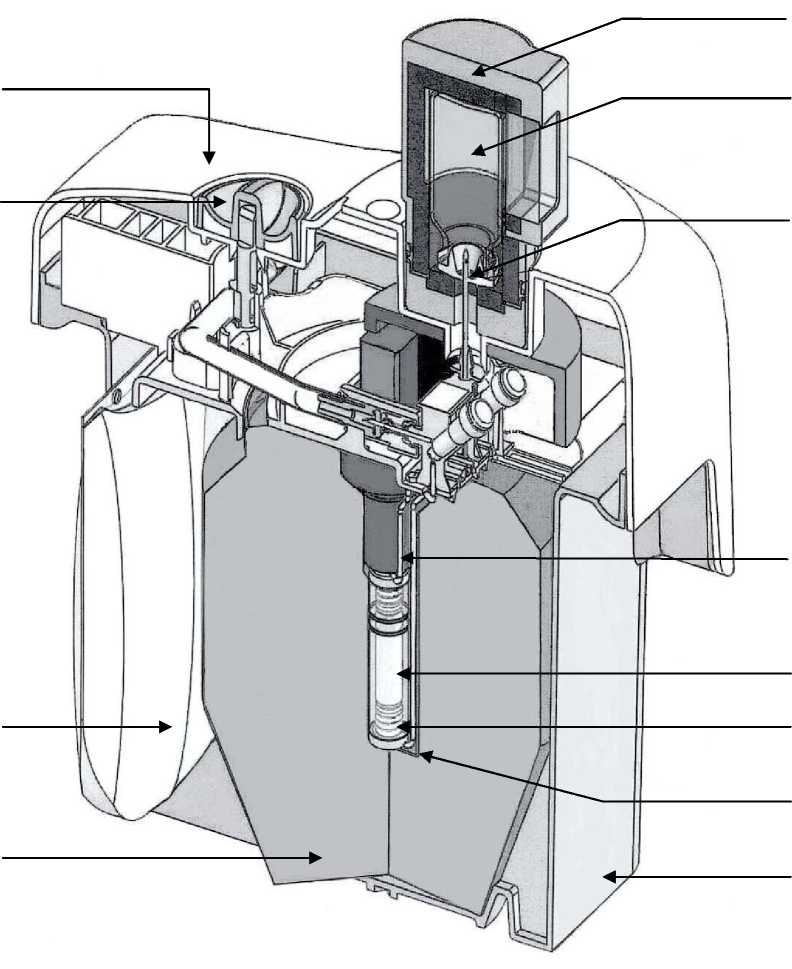
Tekcis generator is delivered in a type A transport container. It comprises:
• a 250 mL soft polypropylene bag containing the elution solution (1). It is connected by a stainless steel needle (2) to the top of the chromatographic column;
• a glass chromatographic column (3) closed at both ends by silicone stoppers filled with sintered stainless steel frits (4). This column contains alumina onto which molybdenum-99 is adsorbed.
• an outlet needle (5) connected to the bottom of the column, while the other end of the needle (6) can be connected to an elution vial to elute the column or a protective vial (STE-ELU) to maintain sterility between two elutions.
The alumina column and needle are protected by cylindro-conical lead or tungsten shielding (7). Generators up to 25 GBq of technetium (99mTc) are protected by lead shielding and that 50 GBq generators are protected by tungsten shielding.
The entire system is placed in a moulded plastic parallelepiped shell (23 x 21 x 14 cm) (8-9).
The elution needle emerges from the upper part of the plastic shell, protected by a transport cap or protective vial (STE-ELU).
A safety valve (10), closed during transport, is situated next to the elution needle.
Accessories supplied with the generator:
• a bag of 10 sterile, pyrogen-free, partial-vacuum elution vials (TC-ELU-5) (11) allowing the elution of 5 mL to 6 mL.
• a sterile elution needle protective vial (STE-ELU).
Each elution vial or protective vial is a 15 mL, colourless, European Pharmacopoeia type I glass vial closed by a rubber stopper and sealed by an aluminium cap.
• An elution container (12) is provided with the first shipment.
Other accessories available:
• kits containing 10 x 15 mL vials:
o partial vacuum vials allowing elution of 5 to 6 mL; o partial vacuum vials allowing elution of 9 to 11 mL; o vacuum vials allowing elution of 14 to 16 mL.
• additional lead shielding adapted to the Tekcis generator: PROTECT ELU.
Packsize :
50 GBq 60 GBq
99mTc activity 2 4 6 8 10 12 16 20 25
(Maximal eluable activity at calibration date, 12h CET)
99Mo activity 2.5 5 7 9.5 12 14.5 19 24 30
(at calibration date, 12h CET)
6.6
6 elution needle
elution container
12
9
10
1
7
11
6
2
3
4
5 8
1 bag of elution solution
2 connection needle
3 glass chromatography column
4 silicone stopper + sintered stainless steel frits
5 stainless steel outlet needle
cylindro-conical lead or tungsten 7
shielding
Special precautions for disposal
General warnings
Radiopharmaceuticals should be received, used and administered only by authorised persons in designated clinical settings. Their receipt, storage, use, transfer and disposal are subject to the regulations and/or appropriate licences of the competent official organisation.
Radiopharmaceuticals should be prepared in a manner which satisfies both radiation safety and pharmaceutical quality requirements. Appropriate aseptic precautions should be taken.
If at any time in the preparation of this product the integrity of this vial is compromised, it should not be used.
Administration procedures should be carried out in a way to minimize risk of contamination of the medicinal product and irradiation of the operators. Adequate shielding is mandatory.
The administration of radiopharmaceuticals creates risks for other persons from external radiation or contamination from spills of urine, vomiting, etc. Radiation protection precautions in accordance with national regulations must therefore be taken.
The residual activity of the generator must be estimated before disposal.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
CIS bio international B.P. 32
F-91192 Gif sur Yvette Cedex
8 MARKETING AUTHORISATION NUMBER(S)
PL-11876/0022
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
30/09/2011
10 DATE OF REVISION OF THE TEXT
07/08/2015
11 DOSIMETRY (IF APPLICABLE)
The data listed below are from ICRP 80 and are calculated according to the following assumptions: (I) Without pre-treatment with a blocking agent:
|
Organ |
Absorbed dose per administered unit of activity (mGy/MBq) | ||||
|
Adults |
15 years |
10 years |
5 years |
1 year | |
|
Adrenal glands |
0.0037 |
0.0047 |
0.0072 |
0.011 |
0.019 |
|
Bladder wall |
0.018 |
0.023 |
0.030 |
0.033 |
0.060 |
|
Bone surfaces |
0.0054 |
0.0066 |
0.0097 |
0.014 |
0.026 |
|
Brain |
0.0020 |
0.0025 |
0.0041 |
0.0066 |
0.012 |
|
Breasts |
0.0018 |
0.0023 |
0.0034 |
0.0056 |
0.011 |
|
Gallbladder |
0.0074 |
0.0099 |
0.016 |
0.023 |
0.035 |
|
Gastrointestinal tract | |||||
|
- Stomach wall |
0.026 |
0.034 |
0.048 |
0.078 |
0.16 |
|
- Small intestine |
0.016 |
0.020 |
0.031 |
0.047 |
0.082 |
|
- Colon |
0.042 |
0.054 |
0.088 |
0.14 |
0.27 |
|
- Ascending colon wall |
0.057 |
0.073 |
0.12 |
0.20 |
0.38 |
|
- Descending colon wall |
0.021 |
0.028 |
0.045 |
0.072 |
0.13 |
|
Heart |
0.0031 |
0.0040 |
0.0061 |
0.0092 |
0.017 |
|
Kidneys |
0.0050 |
0.0060 |
0.0087 |
0.013 |
0.021 |
|
Liver |
0.0038 |
0.0048 |
0.0081 |
0.013 |
0.022 |
|
Lungs |
0.0026 |
0.0034 |
0.0051 |
0.0079 |
0.014 |
|
Muscles |
0.0032 |
0.0040 |
0.0060 |
0.0090 |
0.016 |
|
Oesophagus |
0.0024 |
0.0032 |
0.0047 |
0.0075 |
0.014 |
|
Ovaries |
0.010 |
0.013 |
0.018 |
0.026 |
0.045 |
|
Pancreas |
0.0056 |
0.0073 |
0.011 |
0.016 |
0.027 |
|
Red bone marrow |
0.0036 |
0.0045 |
0.0066 |
0.0090 |
0.015 |
|
Salivary glands |
0.0093 |
0.012 |
0.017 |
0.024 |
0.039 |
|
Skin |
0.0018 |
0.0022 |
0.0035 |
0.0056 |
0.010 |
|
Spleen |
0.0043 |
0.0054 |
0.0081 |
0.012 |
0.021 |
|
Testes |
0.0028 |
0.0037 |
0.0058 |
0.0087 |
0.016 |
|
Thymus |
0.0024 |
0.0032 |
0.0047 |
0.0075 |
0.014 |
|
Thyroid |
0.022 |
0.036 |
0.055 |
0.12 |
0.22 |
|
Uterus |
0.0081 |
0.010 |
0.015 |
0.022 |
0.037 |
|
Other tissue |
0.0035 |
0.0043 |
0.0064 |
0.0096 |
0.017 |
|
Effective dose (mSv/MBq) |
0.013 |
0.017 |
0.026 |
0.042 |
0.079 |
(II) With pre-treatment with a blocking agent:
|
Organ |
Absorbed dose per administered unit of activity (mGy/MBq) when blocking agents are administered | ||||
|
Adults |
15 years |
10 years |
5 years |
1 year | |
|
Organ |
Absorbed dose per administered unit of activity (mGy/MBq) when blocking agents are administered | ||||
|
Adrenal glands |
0.0029 |
0.0037 |
0.0056 |
0.0086 |
0.016 |
|
Bladder wall |
0.030 |
0.038 |
0.048 |
0.050 |
0.091 |
|
Bone surfaces |
0.0044 |
0.0054 |
0.0081 |
0.012 |
0.022 |
|
Brain |
0.0020 |
0.0026 |
0.0042 |
0.0071 |
0.012 |
|
Breasts |
0.0017 |
0.0022 |
0.0032 |
0.0052 |
0.010 |
|
Gallbladder |
0.0030 |
0.0042 |
0.0070 |
0.010 |
0.013 |
|
Gastrointestinal tract | |||||
|
- Stomach wall |
0.0027 |
0.0036 |
0.0059 |
0.0086 |
0.015 |
|
- Small intestine |
0.0035 |
0.0044 |
0.0067 |
0.010 |
0.018 |
|
- Colon |
0.0036 |
0.0048 |
0.0071 |
0.010 |
0.018 |
|
- Ascending colon wall |
0.0032 |
0.0043 |
0.0064 |
0.010 |
0.017 |
|
- Descending colon wall |
0.0042 |
0.0054 |
0.0081 |
0.011 |
0.019 |
|
Heart |
0.0027 |
0.0034 |
0.0052 |
0.0081 |
0.014 |
|
Kidneys |
0.0044 |
0.0054 |
0.0077 |
0.011 |
0.019 |
|
Liver |
0.0026 |
0.0034 |
0.0053 |
0.0082 |
0.015 |
|
Lungs |
0.0023 |
0.0031 |
0.0046 |
0.0074 |
0.013 |
|
Muscles |
0.0025 |
0.0031 |
0.0047 |
0.0072 |
0.013 |
|
Oesophagus |
0.0024 |
0.0031 |
0.0046 |
0.0075 |
0.014 |
|
Ovaries |
0.0043 |
0.0054 |
0.0078 |
0.011 |
0.019 |
|
Pancreas |
0.0030 |
0.0039 |
0.0059 |
0.0093 |
0.016 |
|
Red bone marrow |
0.0025 |
0.0032 |
0.0049 |
0.0072 |
0.013 |
|
Skin |
0.0016 |
0.0020 |
0.0032 |
0.0052 |
0.0097 |
|
Spleen |
0.0026 |
0.0034 |
0.0054 |
0.0083 |
0.015 |
|
Testes |
0.0030 |
0.0040 |
0.0060 |
0.0087 |
0.016 |
|
Thymus |
0.0024 |
0.0031 |
0.0046 |
0.0075 |
0.014 |
|
Thyroid |
0.0024 |
0.0031 |
0.0050 |
0.0084 |
0.015 |
|
Uterus |
0.0060 |
0.0073 |
0.011 |
0.014 |
0.023 |
|
Other tissue |
0.0025 |
0.0031 |
0.0048 |
0.0073 |
0.013 |
|
Effective dose (mSv/MBq) |
0.0042 |
0.0054 |
0.0077 |
0.011 |
0.019 |
The effective dose resulting from the administration of 400 MBq of sodium pertechnetate (99mTc) to an adult weighing 70 kg is about 5.2 mSv.
After pretreatment of patients with a blocking agent and administration of 400 MBq of sodium pertechnetate (99mTc) to an adult weighing 70 kg the effective dose is
1.7 mSv.
The radiation dose absorbed by the lens following administration of sodium pertechnetate (99mTc) for lacrimal duct scintigraphy is estimated to be 0.038 mGy/MBq. This result is an effective dose equivalent of less than 0.01 mSv for an administered activity of 4 MBq.
The specified radiation exposure is only applicable if all organs accumulating sodium pertechnetate (99mTc) will function normally. Hyper/hypofunction (e.g. of the thyroid, gastric mucosa or kidney) and extended processes with impairment to the blood-brain-barrier or renal elimination disorders, may result in changes to the radiation exposure, locally even in the strong increases of it.
External radiation exposure
|
99Mo-99mTc dose rate on the surface of generator (pSv/h.GBq) |
99Mo-99mTc dose rate at 1 m distance from the generator (pSv/h.GBq) | |
|
Shielding with 41 mm lead |
16 |
0.3 |
The surface dose rates and the accumulated dose depends on many factors. Measurements on the location and during work are critical and should be practised for more precise and instructive determination of overall radiation dose to the staff.
12. INSTRUCTIONS FOR PREPARATION OF RADIOPHARMACEUTICALS
• Elution by the generator must be performed in premises complying with the national regulations concerning the safety of use of radioactive products.
• The solution eluted is a clear and colourless sodium pertechnetate (99mTc) solution for injection (Ph. Eur.), with a pH between 4.5 and 7.5 and a radiochemical purity equal to or greater than 95%.
• When sodium pertechnetate (99mTc) solution is used for kit labelling, please refer to the package leaflet of the kit concerned.
• Method of preparation
• Disinfect the stopper of elution vials before each elution.
• Warning:
• Do not use ethanol or ethyl ether to disinfect the stopper of the elution vial, as this may interfere with the elution process.
• During transport, sterility of the elution needle is ensured by a cap.
• Protect the elution needle from possible bacterial contamination by placing the protective vial over the needle between two elutions.
Observe the following sequences to obtain satisfactory results:
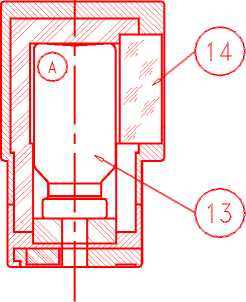
First elution:
When using the generator for the first time, OPEN the safety valve (10) to the ON position BEFORE connecting the elution vial. Never close the safety valve between two elutions. Only close the safety valve at the time of disposal of the generator.
To elute the generator, replace the cap or protective vial by the elution container (A) containing a vacuum elution vial (labelled "TC-ELU") corresponding to the desired elution volume (13).
Elution can be observed through the lead glass window (14) of the container (A). Wait two minutes to ensure complete elution.
Check the clarity of the eluate before use and discard it if the solution is not clear.
• After elution, immediately replace the protective vial to maintain sterility of the needle.
• Elution volumes
• Tekcis is a generator designed to elute all of the available technetium-99m activity in a volume of 5 mL. Fractionated elutions are therefore unnecessary. However, elution to larger volumes can be performed: 10 mL or 15 mL.
• Possibilities of use
• The activity stated on the label of the generator is expressed in technetium-99m available at the calibration date (12:00 CET).
• The available technetium-99m activity depends on:
• the molybdenum-99 activity at the time of elution;
• the time since the last elution.
Quality control
Clarity of the solution, pH, radioactivity and the molybdenum-99 breakthrough must be checked before administration.
The test for molybdenum-99 break-through can be performed either according to Ph. Eur. or to any other validated methods able to determine a molybdenum-99 content below 0.1 per cent of total radioactivity at the date and hour of administration.
Mass of technetium (99mTc + 99Tc) present in the eluate:
Molybdenum-99 is transformed into technetium-99m (87.6% of molybdenum-99 disintegrations) and technetium-99 (12.4% of the molybdenum-99 disintegrations). The total mass of technetium ((99mTc) + (99Tc)) expressed in qg of technetium present in the eluate can be calculated by the following simplified formula:
M(qg) = Technetium-99m activity of the eluate x k F
3
k = 5.161.10- (activity expressed in GBq)
F is the ratio between the number of technetium-99m (N99m) atoms and the total number of technetium atoms (Nt):
F = N99m
Nt
The values of this ratio (F) as a function of the interval between two elutions are presented in the following table:_
|
Hours |
Days | ||||||
|
0 |
1 |
2 |
3 |
4 |
5 |
6 | |
|
0 |
- |
0.277 |
0.131 |
0.076 |
0.0498 |
0.0344 |
0.0246 |
|
3 |
0.727 |
0.248 |
0.121 |
0.072 |
0.0474 |
0.0329 |
0.0236 |
|
6 |
0.619 |
0.223 |
0.113 |
0.068 |
0.0452 |
0.0315 |
0.0227 |
|
9 |
0.531 |
0.202 |
0.105 |
0.064 |
0.0431 |
0.0302 |
0.0218 |
|
12 |
0.459 |
0.184 |
0.098 |
0.061 |
0.0411 |
0.0290 |
0.0210 |
|
15 |
0.400 |
0.168 |
0.092 |
0.058 |
0.0393 |
0.0278 |
0.0202 |
|
18 |
0.352 |
0.154 |
0.086 |
0.055 |
0.0375 |
0.0266 |
0.0194 |
|
21 |
0.311 |
0.141 |
0.081 |
0.052 |
0.0359 |
0.0256 |
0.0187 |
Examples:
The technetium-99m of a generator is eluted into 5 mL; the measured activity is 10 GBq; the previous elution was performed 27 hours earlier.
The mass of technetium is:
M(|ig) = 10 x 5.161.10-3 = 0.208 |ig
0. 248
1. e.: 0.042 |ig/mL
The technetium-99m of a generator is eluted 4 days after preparation (corresponding to the first elution). For an activity of 10 GBq eluted into 5 mL, the mass of technetium is:
M(|ig) = 10 x 5.161.10-3 = 1.036 |ig
0. 0498
1. e.: 0.207 |ig/mL, i.e. five times more technetium than in the previous example. Although small, this amount of technetium may affect the labelling yield of some compounds.
The first eluate obtained from this generator can be normally used, unless otherwise specified. The eluate can be used for kit labelling even eluted after 24 hours from the last elution, except if the use of fresh eluate is specified in the relevant kit SPC.