Teoptic 2% Eye Drops
TEOPTIC® 1% EYE DROPS/ TEOPTIC® 2% EYE DROPS
_(carteolol hydrochloride)_

PACKAGE LEAFLET: INFORMATION FOR THE USER
Teoptic Eye Drops are available using the above names but throughout this leaflet shall be known as Teoptic Eye Drops.
Read all of this leaflet carefully before you start to use this medicine because it contains important information for you.
■ Keep this leaflet. You may need to read it again.
■ If you have further questions, ask your doctor, pharmacist or nurse.
■ This medicine has been prescribed for you only. Do not pass
it on to others. It may harm them, even if their signs of
illness are the same as yours.
■ If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What Teoptic Eye Drops are and what they are used for
2. What you need to know before you use Teoptic Eye Drops
3. How to use Teoptic Eye Drops
4. Possible side effects
5. How to store Teoptic Eye Drops
6. Contents of the pack and other information
1. What Teoptic Eye Drops are and what they are used for
Teoptic Eye Drops are available in two different strengths containing either 1% or 2% of the active ingredient, carteolol hydrochloride.
Carteolol hydrochloride is one of a group of drugs called beta-blockers which can help to reduce pressure in the eye. Teoptic Eye Drops are used to treat conditions such as glaucoma (increased pressure in the eye).
2. What you need to know before you use Teoptic Eye Drops
Do not use Teoptic Eye Drops:
■ if you are allergic to carteolol hydrochloride, beta-blockers or to any of the other ingredients of Teoptic Eye Drops (listed
in section 6).
■ if you have now, or have had in the past, respiratory problems such as asthma, severe chronic obstructive bronchitis (severe lung disease which may cause wheeziness, difficulty in breathing and/or long-standing cough).
■ if you have a slow heartbeat, heart failure or disorders of heart rhythm (irregular heartbeats).
Warnings and precautions
Before you use Teoptic Eye Drops, tell your doctor if you have now, or have had in the past:
■ coronary heart disease (symptoms can include chest pain or tightness, breathlessness or choking), heart failure
■ disturbances of heart rate such as slow heartbeat
■ breathing problems, asthma or chronic obstructive pulmonary disease
■ poor blood circulation disease (such as Raynaud's disease or Raynaud's syndrome)
■ diabetes as carteolol hydrochloride may mask signs and symptoms of low blood sugar
■ overactivity of the thyroid gland as carteolol hydrochloride may mask signs and symptoms
Tell your doctor before you have an operation that you are using Teoptic Eye Drops as carteolol hydrochloride may change effects of some medicines used during anaesthesia.
Other medicines and Teoptic Eye Drops
Teoptic Eye Drops can affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Tell your doctor if you are using or intend to use medicines to lower blood pressure, heart medicine or medicines to treat diabetes.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy, breast-feeding and fertility
Do not use Teoptic Eye Drops if you are pregnant or trying to get pregnant unless your doctor considers it necessary.
Do not use Teoptic Eye Drops if you are breast-feeding. Carteolol hydrochloride may get into your milk.
Ask your doctor for advice before taking any medicine during breast-feeding.
Driving and using machines
Some people may have problems with their eyes such as blurred vision, while they are using Teoptic Eye Drops. If you are affected, you should not drive or use machinery.
Teoptic Eye Drops contain benzalkonium chloride
Benzalkonium chloride may cause eye irritation. Avoid contact with soft lenses. Take out lenses before you use Teoptic Eye Drops and do not put them back for at least 15 minutes. Benzalkonium chloride is known to discolour soft contact lenses.
3. How to use Teoptic Eye Drops
Always use Teoptic Eye Drops exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
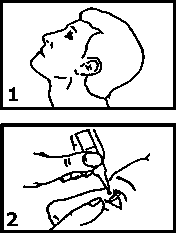
The recommended dose is one drop into the affected eye twice daily. After using Teoptic Eye Drops, press a finger into the corner of your eye, by the nose (picture 3) for 2 minutes. This helps to stop carteolol hydrochloride getting into the rest of the body.
Use in children and adolescents
Teoptic Eye Drops are not recommended for children.
How to use Teoptic Eye Drops
■ Wash your hands before using Teoptic Eye Drops.
■ Remove the cap from the dropper bottle.
■ Make sure that the tip of the bottle does not touch anything.
■ Hold the bottle in one hand between the thumb and forefinger.
■ Tilt your head back, and use your other forefinger to pull down the lower eyelid.
Place the dropper tip close to your eye, but not touching the eye or lid, and gently squeeze the bottle to release one drop into your eye.
Close your eyelid and gently press the corner of your eye with your forefinger for 2 minutes.
■ Replace the cap and wash your hands.
If you forget to use Teoptic Eye Drops
If you forget to use Teoptic Eye Drops, put them in as soon as possible. Do not take a double dose to make up for a forgotten dose.
If you use more Teoptic Eye Drops than you should
If you use too much or if you accidentally swallow Teoptic Eye Drops, see your doctor at once or go to your nearest hospital casualty department. Take your medicine with you.
If you have any further questions on the use of Teoptic Eye Drops, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, Teoptic Eye Drops can cause side effects, although not everybody gets them.
You can usually carry on taking Teoptic Eye Drops, unless the effects are serious. If you are worried, talk to a doctor or pharmacist. Do not stop using Teoptic Eye Drops without speaking to your doctor.
Like other medicines applied into eyes, carteolol hydrochloride is absorbed into the blood. This may cause similar side effects as seen with 'intraveneous' and/or 'oral' as applicable beta-blocking agents. Incidence of side effects after topical ophthalmic administration is lower than when medicines are, for example, taken by mouth or injected. Listed side effects include reactions seen within the class of beta-blockers when used for treating eye conditions:
■ Generalized allergic reactions including swelling beneath the skin that can occur in areas such as the face and limbs, and can obstruct the airway which may cause difficulty swallowing or breathing, hives or itchy rash, localized and generalized rash, itchiness, severe sudden life-threatening allergic reaction.
■ Low blood glucose levels.
■ Difficulty sleeping (insomnia), depression, nightmares, memory loss.
■ Fainting, stroke, reduced blood supply to the brain, increases in signs and symptoms of myasthenia gravis (muscle disorder), dizziness, unusual sensations like pins and needles, and headache.
■ Signs and symptoms of eye irritation (e.g. burning, stinging, itching, tearing, redness), inflammation of the eyelid, inflammation in the cornea, blurred vision and detachment of the layer below the retina that contains blood vessels following filtration surgery which may cause visual disturbances, decreased corneal sensitivity, dry eyes, corneal erosion (damage to the front layer of the eyeball), drooping of the upper eyelid (making the eye stay half closed), double vision, unusual sensitivity to light.
■ Slow heart rate, chest pain, palpitations, oedema (fluid build up), changes in the rhythm or speed of the heartbeat, congestive heart failure (heart disease with shortness of breath and swelling of the feet and legs due to fluid build up), a type of heart rhythm disorder, heart attack, heart failure.
■ Low blood pressure, Raynaud's phenomenon, cold hands and feet.
■ Constriction of the airways in the lungs (predominantly in patients with pre-existing disease), difficulty breathing, cough.
■ Taste disturbances, nausea, indigestion, diarrhoea, dry mouth, abdominal pain, vomiting.
■ Hair loss, skin rash with white silvery coloured appearance (psoriasiform rash) or worsening of psoriasis, skin rash.
■ Muscle pain not caused by exercise.
■ Sexual dysfunction, decreased libido.
■ Muscle weakness/tiredness, feeling of discomfort, feeling of tension or fullness in the nose, cheeks and behind your eyes, sometimes with a throbbing ache, fever, stuffy nose and loss of the sense of smell (sinusitis).
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via:
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Teoptic Eye Drops
■ KEEP OUT OF THE SIGHT AND REACH OF CHILDREN.
■ Teoptic Eye Drops are sterile until the seal is broken.
■ Do not use if the seal is broken when you receive the bottle.
■ Do not use Teoptic Eye Drops later than one month after first breaking the seal.
■ Do not use after the expiry date shown on the label. The expiry date refers to the last day of that month.
■ Do not store above 25°C.
■ If your doctor decides to stop the treatment, return any leftover eye drops to the pharmacist. Only keep them if your doctor tells you to.
■ If your eye drops appear discoloured or show any other signs of deterioration, take them to your pharmacist who will advise you.
■ Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information
What Teoptic Eye Drops contain
Teoptic Eye Drops contain a medicine called carteolol.
Teoptic Eye Drops also contain benzalkonium chloride (as a preservative) and sodium chloride, sodium phosphate monobasic and dibasic and water.
What Teoptic Eye Drops look like and contents of the pack
Teoptic Eye Drops come in plastic dropper bottles with a plastic cap and a tamper evident seal containing a clear colourless solution.
Teoptic Eye Drops come in two strengths, containing 10mg or 20mg of carteolol hydrochloride. 1x5ml bottle contains 5ml of eye drops.
Manufacturer
Your medicine is manufactured by: Otsuka Pharmaceutical s.a., Barcelona, Spain.
Or
Otsuka Pharmaceutical s.a., Avda. Diagonal, 609-615, 08028, Barcelona, Spain.
Procured from within the EU and repackaged by: Doncaster Pharmaceuticals Group Ltd., Kirk Sandall, Doncaster, DN3 1QR.
Product Licence holder: Landmark Pharma Ltd., 7 Regents Drive, Prudhoe, Northumberland, NE42 6PX.
PL No: 21828/0105 1% IT—
PL No: 21828/0106 2% POM
Leaflet revision date: 23.01.15
TEOPTIC® is a registered trademark of Otsuka Pharmaceutical Co. Ltd.
If you wish to receive this leaflet in Braille, large font or audio format please contact 01302 365000 and ask for the Regulatory Department.
Page 2 of 2