Tesco Hayfever 2% Eye Drops
PACKAGE LEAFLET: INFORMATION FOR THE USER
Read all of this leaflet carefully before you start using this medicine.
This medicine is available without a prescription, however you still need to use this medicine carefully to get the best results from it.
• Keep this leaflet. You may need to read it again.
• Ask your pharmacist if you need more information or advice.
• You must contact a doctor if your symptoms worsen or do not improve after 14 days.
• If any of the side effects get serious, or you notice any side effects not listed in this leaflet, please tell your pharmacist.
IN THIS LEAFLET:
1. What this medicine is for
2. Before you use this medicine
3. How to use this medicine
4. Possible side effects
5. How to store this medicine
6. Further information
Q WHAT THIS MEDICINE IS FOR
Sodium cromoglicate belongs to a group of medicines called anti-allergies. Sodium cromoglicate is used for the relief and treatment of the eye symptoms of hayfever. It works by reducing the body's response to the allergy when used regularly during the course of treatment.
These symptoms occur usually in the late spring or early summer when pollen from grasses or trees comes into contact with the eyes of people who are sensitive to them. This condition is sometimes called ‘hayfever eyes’ or by its medical term, seasonal allergic conjunctivitis.
If you suffer from hayfever, you can also help control your symptoms by trying to ensure low exposure to pollen when hayfever symptoms are expected. For example, keep windows closed, do not cut grass, stay indoors as much as possible.
L
□ BEFORE YOU USE THIS MEDICINE
Do not use:
• In children under 6 years of age.
• If you or your child are sensitive to eye drops containing sodium cromoglicate, benzalkonium chloride or disodium edetate.
You must be confident that you or your child’s eye symptoms are caused by hayfever, or have been previously diagnosed by your doctor to suffer from hayfever: You can be sure allergic eyes are caused by hayfever if:
• Both eyes are affected • You also have a runny or blocked nose
• Your eyesight is not affected • You know you suffer from hayfever and your symptoms coincide with the times of year when grass or tree pollen is high. But if you or your child have:
• No nose symptoms • Only one eye is affected • Your sight is affected Or if the eye symptoms:
• occur at the wrong time of year for hayfever • are likely to be caused by another allergy, e.g. a pet or dust mite allergy then you cannot be sure that the condition is due to hayfever and you should see your doctor or pharmacist before you use these drops.
Talk to your doctor or pharmacist before use:
• If you are pregnant or breast feeding.
Important information about some of the ingredients of this medicine
This product contains benzalkonium chloride. This may cause eye irritation. Benzalkonium chloride is known to discolour soft contact lenses. Avoid contact with soft contact lenses. For all other types of contact lenses: Remove contact lenses prior to application and wait at least 15 minutes before reinsertion.
Driving and using machinery
This medicine can cause blurred vision for a few minutes after use.
If this happens, do not drive or operate machinery until you can see normally again.
Further information overleaf ^
HOW TO USE THIS MEDICINE ^our e^es maT a jusFafterH
you use the drops. If they sting badly, or if your eyes get worse, stop using the drops and tell your doctor or pharmacist.
You may also experience blurred vision for a short time after application. If you notice any other side effects, tell your doctor or pharmacist.
For external use only.
Adults (including the elderly) and children over six years:
Place one or two drops into each eye four times daily.
Wash your hands before using the drops.
Tilt your head back and carefully pull down your lower eyelid.
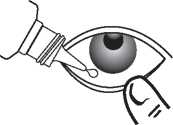
If any of the side effects get serious or you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
HOW TO STORE THIS MEDICINE
Without touching the eyelid with the tip of the dropper, put one or two drops into your lower lid and then blink a few times to make sure your whole eye is covered by the liquid. Replace the cap securely on the bottle.
If you have any questions concerning the use of these drops in a child, or are unsure if the child has eye symptoms of hayfever, you should seek advice from your doctor or pharmacist.
Remember: Keep all medicines out of the reach and sight of children.
Replace cap securely after use.
Store the eye drops below 25°C. Keep the bottle in the outer carton to protect from light.
Do not use the eye drops after the expiry date printed on the label.
Do not use the eye drops more than four weeks after first opening the bottle. Discard any unused medicine after this time.
If your eyes are no better after 2 days of using the eye drops, see your doctor or pharmacist.
If you find you need to use the drops continuously for more than 14 days, then check with your pharmacist or doctor before continuing.
If you use more of this medicine than you should
[Contact your doctor if you use the eye drops more frequently than the [recommended dose.
If you forget to use this medicine
|lf you miss a dose, use the drops as directed above and then continue k'our normal course of treatment.
Do not use a double dose to make up for a forgotten dose.
n POSSIBLE SIDE EFFECTS
Like all medicines, these drops can cause side effects, although not everybody gets them. All medicines can cause allergic reactions, although serious allergic reactions are very rare. [Tell your doctor straight away if you get any sudden wheeziness,
[difficulty in breathing, swelling of the eyelids, face or lips, rash, or itching [especially affecting your whole body).
Medicines should not be disposed ofi via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
HI FURTHER INFORMATION
What your medicine contains
The active ingredient in this medicinel is sodium cromoglicate 2% w/v.
The other ingredients are disodium edetate, benzalkonium chloride and water.
What this medicine looks like and contents of the pack
This medicine is a clear liquid available in a tamper-evident bottle fitted with ai dropper and closed with a screw capJ The bottle contains 5 or 10ml of drops..
Marketing Authorising Holder:
PLIVA Pharma Ltd, Ridings Point, Whistler Drive, Castleford,
WF10 5HX, UK.
Manufacturer:
Alcon Cusi SA, C/Camil Fabra,
58 El Masnou, Barcelona, Spain.
This leaflet was last revised: September 2011
4002741 _ _ _ 01021-B