Testogel 16.2 Mg/G Gel
What is in this leaflet:
1. What TESTOGEL® 16.2 mg/g is and what it is used for
2. What do you need to know before you use TESTOGEL® 16.2 mg/g
3. How to use TESTOGEL® 16.2 mg/g
4. Possible side effects
5. How to store TESTOGEL® 16.2 mg/g
6. Contents of the pack and other information
1. WHAT TESTOGEL® 16.2 MG/G IS AND WHAT IT IS USED FOR
This medicine contains testosterone, a male hormone produced naturally in the body.
TESTOGEL® 16.2 mg/g is used in adult men for testosterone replacement to treat various health problems caused by a lack of testosterone (male hypogonadism). This should be confirmed by two separate blood testosterone measurements and also include clinical symptoms such as: impotence infertility low sex drive tiredness
depressive moods
bone loss caused by low hormone levels
2. WHAT YOU NEED TO KNOW BEFORE YOU USE TESTOGEL® 16.2 MG/G Do not use TESTOGEL® 16.2 mg/g
- if you have or are suspected of having prostate cancer
- if you have or are suspected of having breast cancer
- if you are allergic to testosterone or any of the other ingredients of this medicine (listed in section 6)
Warnings and precautions
Talk to your doctor or pharmacist before using TESTOGEL® 16.2 mg/g.
Before starting any treatment with TESTOGEL® 16.2 mg/g, your testosterone deficiency must be clearly demonstrated by clinical signs (regression of masculine characteristics, reduced lean body mass, weakness or fatigue, reduced sexual desire/drive, inability to have/maintain an erection, etc.) and confirmed by laboratory tests.
If you are arranging for testing of blood samples while using TESTOGEL® 16.2 mg/g you must ensure that all testosterone measurements are performed in the same laboratory, because of the variability in analytical values among diagnostic laboratories.
TESTOGEL® 16.2 mg/g is not recommended for:
- the treatment of male sterility or impotence
- children, as no clinical information is available for boys under 18 years
Androgens may increase the risk of an enlarged prostate gland (benign prostatic hypertrophy) or prostate cancer. Regular examinations of the prostate gland before the beginning and during therapy should be performed to follow your doctor's recommendations.
If you are suffering from severe heart, liver or kidney disease, treatment with TESTOGEL® 16.2 mg/g may cause severe complications in the form of water retention in your body, sometimes accompanied by congestive heart failure (fluid overload in the heart).
The following blood checks should be carried out by your doctor before and during the treatment: testosterone blood level, full blood count.
Tell your doctor if you have high blood pressure or if you are treated for high blood pressure, as testosterone may cause a rise in blood pressure. TESTOGEL® 16.2 mg/g should therefore be used with caution if you are suffering from high blood pressure.
Worsening of breathing problems during sleep have been reported during treatment with testosterone in some people, particularly those who are very overweight or are already suffering from breathing difficulties.
If you have cancer that affects your bones, you may develop increased levels of calcium in your blood or urine. TESTOGEL® 16.2 mg/g may further affect these calcium levels. Your doctor may wish to check these calcium levels using blood tests during your treatment with TESTOGEL® 16.2 mg/g.

Package Leaflet: Information for the Patient
TESTOGEL® 16.2 mg/g Gel
Testosterone
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
If you receive testosterone replacement treatment over long periods of time you may develop an abnormal increase in the number of red blood cells in your blood (polycythaemia). You will need regular blood tests to check that this is not happening.
TESTOGEL® 16.2 mg/g must be used with caution if you are suffering from epilepsy and/or from migraine as these conditions may worsen.
If have diabetes and use insulin to control your blood sugar levels, testosterone treatment may affect your response to insulin and your antidiabetics' medication might need adjustment.
In case of severe skin reactions, treatment should be reviewed and stopped if necessary.
The following signs may indicate you are taking too much of the product: irritability, nervousness, weight gain, frequent or prolonged erections. Report any of these to your doctor who will adjust the daily dose of TESTOGEL® 16.2 mg/g.
Before starting treatment your doctor will perform a full examination. He/she will need to take blood samples on two visits to measure your levels of testosterone before you are given this medicine. You will have regular check-ups (at least once a year and twice a year if you are elderly or an at risk patient) during treatment.
TESTOGEL® 16.2 mg/g should not be used by women due to possible virilising effects (such as growth of facial or body hair, deepening of the voice or changes in the menstrual cycle).
Sportsmen and women
The attention of sportsmen and women is drawn to the fact that this proprietary medicine contains an active substance (testosterone) that may produce a positive result in doping control tests.
Possible transfer of testosterone
During close and relatively long periods of skin contact testosterone may be transferred to another person unless you cover the treated area. This could cause your partner to show the signs of increased testosterone, such as more hair on the face and body and a deepened voice. It may cause changes in the menstrual cycle of women, premature puberty and genital enlargement in children. Wearing clothes covering the application area or showering before contact protects against such transfer.
The following precautions are recommended:
- wash your hands with soap and water after applying the gel
- cover the application area with clothing once the gel has dried
- take a shower before intimate contact or if not possible wear clothing such as a shirt or a T-shirt covering the application site during the contact period
- wear clothing (such as a sleeved shirt) covering the application site during contact periods with children
If you believe testosterone has been transferred to another person (woman or child)
- wash the area of skin that may have been affected immediately with soap and water
- report any signs such as acne or changes in the growth or pattern of hair on your body or face to your doctor
You should preferably wait at least 2 hours between applying the gel and taking a bath or shower. If you occasionally need to bath or shower between 2 and 6 hours after applying the gel it should not significantly change the effects of your treatment.
In order to improve the safety of your partner you should wash the area with soap for instance during a shower before having sexual intercourse or, if not possible, wear a T-shirt that covers the application site at the time of contact.
You should wear a T-shirt that covers the application site when in contact with children in order to avoid the risk of transferring the gel to the child's skin.
Other medicines and TESTOGEL® 16.2 mg/g
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, particularly the following:
- oral anticoagulants (used to thin the blood)
- corticosteroids
These particular medicines may lead to your dose of TESTOGEL® 16.2 mg/g being adjusted.
Pregnancy, breast-feeding and fertility
TESTOGEL® 16.2 mg/g is not to be used by pregnant or breast-feeding women.
Pregnant women must avoid any contact with the TESTOGEL® 16.2 mg/g application sites. This medicine may cause the development of unwanted male characteristics in the developing baby. In the event of contact, as recommended above, wash the area of contact as soon as possible with soap and water.
If your partner becomes pregnant you must follow the advice regarding avoiding transfer of the testosterone gel.
Spermatozoid production may be reversibly suppressed with TESTOGEL® 16.2 mg/g.
XXXXXXXXXXXX
Driving and using machines
TESTOGEL® 16.2 mg/g has no influence on the ability to drive or use machines.
3. HOW TO USE TESTOGEL® 16.2 MG/G This medicine is for use by adult men only.
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Using the pump for the first time
Before using your new pump for the first time, you need to prepare it for use as follows:
- Remove the cap from the canister to reveal the plunger
- Remove the stopper from the spout
- Press the plunger down three times
- Do not use the gel from these three depressions of the plunger. This dose should be safely discarded.
- Your pump is now ready to use. You will not need to prime it again
- Each time the plunger is depressed it delivers 1.25 g of gel
The recommended dose is 2.5 g of gel (/.e. 40.5 mg of testosterone) applied once daily at approximately the same time each day, preferably in the morning. To obtain 2.5 g of gel the plunger should be depressed twice. The dose may be adjusted by your doctor, and the maximum dose is 5 g of gel per day (no more than four depressions of the plunger).
Your doctor will tell you how many depressions of the plunger to make to get the correct dose of gel for you. The table below gives you more information on this.
|
Number of Depressions |
Amount of Gel (g) |
Quantity of Testosterone Applied to the Skin (mg) |
|
1.25 |
20.25 | |
|
2 |
2.5 |
40.5 |
|
3 |
3.75 |
60.75 |
|
4 |
5.0 |
81.0 |
Ireland Marketing Authorisation Holder Laboratoires Besins International 3 rue du Bourg l'Abbe 75003 Paris France
UK Marketing Authorisation Holder Besins Healthcare Avenue Louise 287 1050 Brussels Belgium
Manufacturer (UK and Ireland)
Laboratoires Besins International 13, rue Perier 92120 Montrouge France
Distributed in the UK and in Ireland by
Besins Healthcare (UK) Ltd
35A High Street
Marlborough - SN8 1LW
United Kingdom
Tel. +44(0)1672 516885
This medicinal product is authorised in the Member States of the EEA under the following names:
Austria, Belgium, France, Iceland, Italy, Luxembourg, Romania, Spain, The Netherlands, Czech Republic, Hungary: Androgel 16,2 mg/g Denmark: Androgel
Germany, Ireland, United Kingdom: Testogel 16.2 mg/g Finland: Androtopic 16,2 mg/g Poland: Androtop
Slovenia: Androtop 20,25 mg/sprozitev gel
For information in large print, tape, CD or Braille, telephone +44(0)1672 516885.
This leaflet was last revised in May 2016.
XXXXXXXXXXXX
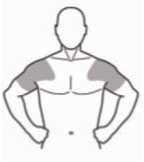
The gel must be gently spread onto clean, dry, healthy skin as a thin layer on both shoulders and upper arms. Do not rub it into the skin. Allow the gel to dry for at least 3-5 minutes before dressing. Wash your hands with soap and water after applying. Do not apply to the genital areas (penis and testes) as the high alcohol content may cause
local irritation.
If you use more TESTOGEL® 16.2 mg/g than you should
Seek advice from your doctor or pharmacist. Treatment of overdose would consist of discontinuation of TESTOGEL® 16.2 mg/g together with appropriate symptomatic and supportive care.
If you forget to use TESTOGEL® 16.2 mg/g
Do not use a double dose to make up for a forgotten individual dose. Apply the next dose at the usual time.
If you stop using TESTOGEL® 16.2 mg/g
You should not stop therapy with TESTOGEL® 16.2 mg/g unless told to do so by your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them:
Common side effects (may affect up to 1 in 10 people)
TESTOGEL® 16.2 mg/g may cause changes to your mood (including mood swings, anger or aggression, impatience, sleeplessness, abnormal dreams and increased sex drive) and skin reactions (including acne, hair loss, dry skin, skin irritation, hair colour changes, rash and sensitive skin), increase in red blood cell count, haematocrit (percentage of red blood cells in blood) and haemoglobin (the component of red blood cells that carries oxygen), identified by periodic blood tests and changes to the prostate gland (including increased blood levels of a protein called prostate specific antigen that is produced by the prostate).
Uncommon side effects (may affect up to 1 in 100 people)
TESTOGEL® 16.2 mg/g may cause an increase in blood pressure, flushing,
inflammation of the veins, diarrhoea, bloated stomach, pain in the mouth, development of breasts, tender nipples, pain in the testicles, fluid retention.
Other side effects have been observed during treatment with TESTOGEL® 16.2 mg/g : tiredness, depression, anxiety, headache, dizziness, tingling skin, blood clots, difficulty breathing, feeling sick, sweating, abnormal body hair growth, muscle or bone pain, difficulty passing urine, reduced number of sperm, muscle weakness, feeling unwell, weight gain.
Because of the alcohol this medicine contains, frequent applications to the skin may cause irritation and skin dryness.
Reporting of side effects
United Kingdom
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme.
Website: www.mhra.gov.uk/yellowcard Ireland
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2;
Tel: +353 1 6764971; Fax: +353 1 6762517.
Website: www.hpra.ie; E-mail: medsafety@hpra.ie.
By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE TESTOGEL® 16.2 MG/G
This medicinal product does not require any special storage conditions.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the labelling after EXP.
6. CONTENTS OF THE PACK AND OTHER INFORMATION What TESTOGEL® 16.2 mg/g contains
- The active substance is testosterone.
- The other ingredients are Carbomer 980, isopropyl myristate, ethanol 96%, sodium hydroxide, purified water.
What TESTOGEL® 16.2 mg/g looks like and contents of the pack
TESTOGEL® 16.2 mg/g is a colourless gel presented in a multi-dose container with metering pump with 88 g gel and delivers a minimum of 60 doses.
One gram of gel contains 16.2 mg testosterone. One pump actuation delivers 1.25 g of gel containing 20.25 mg of testosterone.
TESTOGEL® 16.2 mg/g is available in packs containing one, two, three or six containers. Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Besins Manufacturing Belgium GrootBijgaardenstraat 128 1620 Drogenbos Belgium
