Thwart 26%W/W Cutaneous Solution
ALLIANCE
Thwart® 26%w/w cutaneous solution
salicylic acid
Information for Patients
Read all of this leaflet carefully because it contains important information for you.
This medicine is available without prescription. However, you still need to use Thwart carefully to get the best results from it.
• Keep this leaflet. You may need to read it again.
• Ask your pharmacist if you need more information or advice.
• You must contact a doctor, pharmacist or nurse if your symptoms worsen or do not improve.
• If any of the side effects gets serious, or if you notice any side effect not listed in this leaflet, please tell your doctor, pharmacist or nurse.
The information in this leaflet has been divided into the following sections:
1. What Thwart is and what it is used for
2. Check before you use Thwart
3. How to use Thwart
4. Possible side effects
5. How to store Thwart
6. Further information
1. What Thwart is and what it is used for
Thwart is used to treat and remove raised warts (usually found on the hands, elbows or knees) and verrucas (warts on the feet). Thwart is a special solution which can be easily applied to the wart, dries quickly, needs no plasters and if used properly should cause minimal irritation.
Warts, or verrucas as they are often called, are found most commonly on the hands, feet and knees. They are commonest amongst children of school age.
Warts are caused by a virus which gets into the upper layers of the skin. The body then reacts to the virus, which leads to the formation of the wart or verruca. Warts are most frequently transmitted by contact, e.g. swimming pools and communal changing rooms are often associated with infection. Warts can occur singly or in groups and can be transferred from one part of the body to another, e.g. from one foot to the other.
Thwart contains a high concentration of salicylic acid which when used regularly removes the dead cells from the outer layer of the skin and steadily reduces the size of the wart.
Do not use Thwart:
• if you are allergic (hypersensitive) to salicylic acid (aspirin) or any of the other ingredients of Thwart (see section 6 Further information)
• if you have diabetes
• if you have poor blood circulation
• if the wart or skin surrounding it is inflamed or broken
• on moles, birthmarks, unusual warts with hairgrowth
• on your face
• on warts in the anal or genital region.
Take special care with Thwart
Thwart is flammable - keep it away from flames and fire.
Thwart is for external use only and should only be applied to warts.
Do not use on broken or damaged skin such as cuts or grazes.
Try not to get Thwart on the normal skin around the wart. You should apply Thwart to the centre of the wart therefore avoiding the surrounding healthy skin.
Do not allow Thwart to come into contact with mucous membranes (e.g. your mouth, nose and other body openings) or your eyes.
If Thwart gets into any of these areas, flush immediately with water for 15 minutes.
Using other medicines
When used according to the instructions, Thwart is not known to interfere with any other medicines. However, you should not use any other treatments on your warts or verrucas at the same time as using Thwart.
Pregnancy and breast-feeding
The safe use of Thwart during pregnancy and breast-feeding has not been established. Therefore Thwart should be used with caution or following advice of your doctor.
Ask your doctor or pharmacist for advice before taking any medicine.
3. How to use Thwart
Apply Thwart once daily as follows:
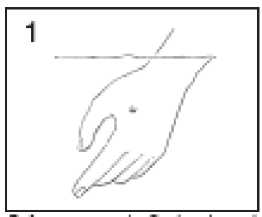
Before you apply Thwart, soak affected areas in warm water for 5 minutes.
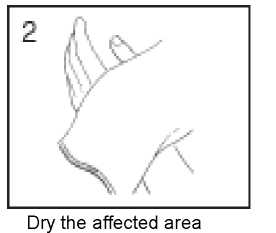
thoroughly using a towel. The towel should not be used by anyone else otherwise the wart could be passed to them.
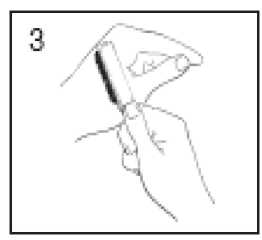
Gently remove any loose tissue from the wart surface by rubbing with a brush, emery board, pumice or abrasive sponge. Be careful not to damage the surrounding healthy skin.
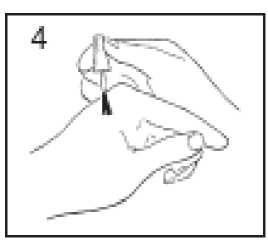
Use the applicator brush supplied in the cap to apply Thwart to the wart. Do not get any on the surrounding healthy skin. Allow Thwart to dry completely and then apply a second coat. You do not need to cover the wart with a plaster or bandage.
You should notice an improvement within 1-2 weeks but for maximum effect, continue treatment for 4-6 weeks.
Do not allow the solution to drip onto the neck of the bottle or you may find it difficult to open the bottle next time.
What to do if you use more Thwart than you should
If you accidentally swallow Thwart, use too much of it or use it for long periods of time, the body could absorb too much salicylic acid. This could cause ringing in the ears, feeling or being sick. If this occurs, or you notice any other symptoms, stop using Thwart and consult your doctor.
If you forget to use Thwart
If you forget to use Thwart on any occasion, do not worry, just continue to use daily as before.
If you have any further questions on the use of this product, ask your doctor, nurse or pharmacist.
Do not worry. Like all medicines, Thwart can cause side effects, although not everyone gets them.
If any of the following symptoms occur you should stop using Thwart and contact your doctor or pharmacist:
• ringing in the ears
• feeling or being sick.
Other side effects include:
• redness, rash or itching affecting the normal skin around the wart. If this occurs, stop using Thwart for a few days until the irritation goes away. When you re-start treatment, be careful to apply the solution only to the wart itself.
If Thwart causes a lot of skin irritation, stop using it and contact your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor, nurse or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via:
UK
The Yellow Card Scheme: www.mhra.gov.uk/yellowcard.
IE
HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2;
Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie, E-mail: medsafety@hpra. ie.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Thwart
Thwart is flammable - keep away from fire or flame.
Keep out of the reach and sight of children.
Do not use Thwart after the expiry date which is stated on the carton and bottle. The expiry date refers to the last day of that month after EXP.
Do not store Thwart above 25°C. Keep bottle tightly closed when not in use and avoid dripping the solution on to the thread of the bottle-neck which can make opening difficult.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist on how to dispose of medicines no longer required. These measures will help protect the environment.
6. Further information
What is in Thwart?
The active ingredient in this medicine is salicylic acid 26% w/w.
The other ingredients are polyvinyl butyral, dibutyl phthalate, isopropyl alcohol, butyl acetate and acrylates copolymer.
What Thwart looks like and contents of the pack
Thwart is a colourless to pale yellow solution.
Thwart is available in amber, glass bottles containing 10 millilitres, with an applicator brush in the cap.
Marketing Authorisation Holder and Manufacturer
The Marketing Authorisation Holder is: Alliance Pharmaceuticals Ltd, Avonbridge House, Chippenham, Wiltshire, SN15 2BB, UK.
Manufactured by Pharmasol Ltd, North Way, Andover, Hants, SP10 5AZ, UK and Biotec (Suffolk) Ltd, Great Yarmouth, Norfolk, NR30 3DN, UK.
The information in this leaflet applies only to Thwart. If you have any questions or you are not sure about anything, ask your doctor or a pharmacist.
This leaflet was last approved: 5th December 2014
Thwart is a registered trademark of Alliance Pharmaceuticals Limited.
Alliance and associated devices are registered trademarks of Alliance Pharmaceuticals Limited.
© Alliance Pharmaceuticals Ltd 2014
Thwart PIL UK ROI 003