Timolol 0.25 % W/V Eye Drops Solution
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER
Timolol 0.25% w/v Eye Drops, Solution Timolol 0.5% w/v Eye Drops, Solution (timolol maleate)
(referred to as Timolol Eye Drops in the rest of this leaflet)
Read all of this leaflet carefully before you start using this medicine
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Timolol Eye Drops are and what they are used for
2. Before you use Timolol Eye Drops
3. How to use Timolol Eye Drops
4. Possible side effects
5. How to store Timolol Eye Drops
6. Further information
1. WHAT TIMOLOL EYE DROPS ARE AND WHAT THEY ARE USED FOR
Timolol Eye Drops are available in two strengths: Timolol 0.25% w/v Eye Drops, Solution and Timolol 0.5% w/v Eye Drops, Solution.
The active ingredient in Timolol Eye Drops is timolol maleate, which reduces the pressure inside the eyeball.
Timolol Eye Drops is a member of a group of medicines known as beta-blockers and it is used to reduce the pressure inside your eyeball(s) if you have a condition such as high blood pressure that is affecting your eyes, or glaucoma (high pressure in your eyeball), including glaucoma that developed after you had surgery to remove a cataract from your eye(s).
2. BEFORE YOU USE TIMOLOL EYE DROPS
Do not use Timolol Eye Drops
• if you are allergic (hypersensitive) to timolol, beta-blockers or any of the other ingredients in Timolol Eye Drops (listed in the section of this leaflet “What Timolol Eye Drops contain")
• If you know you are allergic (hypersensitive) to another type of beta-blocker such as propranolol (used mainly for high blood pressure) or betaxolol (also used for glaucoma)
• if you have now or have had in past respiratory problems such as asthma, severe chronic obstructive bronchitis, (severe lung disease which may cause wheeziness, difficulty in breathing and/or long-standing cough)
• if you have a slow heart beat or heart block (a problem with the electrical conduction system of your heart)
• if you have heart failure, including heart failure accompanied by low blood pressure.
Take special care with Timolol Eye Drops
Paediatric population:Timolol eye drop solution should generally be used with caution in young patients. In newborns, infants and younger children Timolol should be used with extreme caution. If coughing, wheezing, abnormal breathing or abnormal pauses in breathing (apnoea) occur, the use of the medication would be stopped immediately. Contact your doctor as soon as possible. A portable apnoea monitor may also be helpful.
Timolol has been studied in infants and children aged 12 days to 5 years, who have raised pressure in the eye(s) or have been diagnosed with glaucoma. For more information talk to your doctor.
Before you use this medicine, tell your doctor if you have now or have had in the past
• if you have ever had coronary heart disease, symptoms can include chest pain or tightness, breathlessness or choking, heart failure, low blood pressure (hypotension)
• disturbances of heart rate such as slow heart beat (bradycardia).
• breathing problems, asthma or chronic obstructive pulmonary disease (lung disease which may cause wheeziness, difficulty in breathing and/or long-standing cough)
• poor blood circulation disease (peripheral arterial disease such as Raynaud's disease or Raynaud's syndrome)
• diabetes as timolol may mask signs and symptoms of low blood sugar
• overactivity of the thyroid gland as Timolol may mask signs and symptoms
• if you are already taking a different type of beta-blocker such as propranolol (used mainly for high blood pressure) or betaxolol (which is also an eye drop used for glaucoma)
• if you wear soft contact lenses (see the section of this leaflet “Important information about some of the ingredients of Timolol Eye Drops") - Hard contact lenses may generally be worn without problems whilst taking Timolol Eye Drops
• if you have had an eye injury, infection or surgery
• if you have a type of glaucoma called ‘closed angle glaucoma’
• if you have any other disease or condition affecting your eye(s)
• if you have ever had an allergy, such as hay fever, or a bad allergic reaction to something (symptoms of an allergic reaction can include warmth and redness of the skin, a rash on most or part of the body, a swollen tongue or throat or difficulty breathing), since you might get a severe allergic reaction when you use Timolol Eye Drops repeatedly
• If you ever have drug tests as Timolol Eye Drops may produce positive results.
If any of these points apply to you, talk to your doctor or pharmacist before you use Timolol Eye Drops.
Tell your doctor or pharmacist immediately if:
• you suffer an eye injury, have eye surgery or get an eye infection whilst you are using Timolol Eye Drops
• a rash develops on your skin or your eyes become dry whilst you are using Timolol Eye Drops.
Tell your doctor before you have an operation that you are using Timolol Eye Drops as timolol maleate may change effects of some medicines used during anaethesia.
Using other medicines
Timolol Eye Drops can affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Tell your doctor if you are using or intend to use medicines to lower blood pressure, heart medicine or medicines to treat diabetes. Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Please tell your doctor or pharmacist if you are taking or have recently taken any of the following medicines before you use Timolol Eye Drops:
• Adrenaline, used for conditions including asthma and allergic reactions
• Digoxin, used mainly for heart failure or an abnormal heart rhythm
• Quinidine, used to treat heart conditions and some types of malaria
• Fluoxetine and paroxetine, used to treat depression
• Calcium channel blockers such as nifedipine, verapamil or diltiazem, used mainly for angina or high blood pressure
• Clonidine or reserpine, used mainly for high blood pressure
• Other beta-blockers such as propanolol, acebutalol, atenolol, bisoprolol, carvedilol, celiprolol, labetolol, metoprolol, nadolol, nebivolol, oxprenalol, pindolol or sotalol.
Pregnancy and breast-feeding
Do not use Timolol Eye Drops if you are pregnant unless your doctor considers it necessary.
Do not use Timolol Eye Drops if you are breast-feeding. Timolol maleate may get into your milk.
Ask your doctor or pharmacist for advice, before using Timolol Eye Drops or any other medicinal product, if you are pregnant, think you are pregnant, are trying to get pregnant or are breastfeeding a baby.
Driving and using machines
Timolol Eye Drops may cause side effects such as dizziness and problems with vision, which may affect your ability to drive or to use tools and machines.
Do not drive or operate tools and machines whilst you are using Timolol Eye Drops unless you have talked to your doctor or pharmacist and asked for their advice first.
Important information about some of the ingredients of Timolol Eye Drops Timolol Eye Drops contain benzalkonium chloride. This may cause eye irritation.
Avoid contact with soft contact lenses. Remove contact lenses prior to applying Timolol Eye Drops to your eye(s) and wait for at least 15 minutes before reinsertion. Timolol Eye Drops are known to discolour soft contact lenses.
Hard contact lenses may generally be worn without problems whilst taking Timolol Eye Drops.
3. HOW TO USE TIMOLOL EYE DROPS
Always use Timolol Eye Drops solution exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Always wash your hands before using Timolol Eye Drops.
Timolol Eye Drops are for ocular use only (to be used as eye drops only) - do not swallow.
Posology
Paediatric population
A detailed medical examination should precede the use of Timolol. Your doctor will carefully evaluate the risks and benefits when considering treatment with Timolol. If the benefits outweigh the risks, it is recommended to use the lowest active agent concentration available once daily. With regard to "the use in children', the 0,1% active agent concentration may be sufficient to control pressure within the eye. If the pressure is not sufficiently controlled with the dosage, a twice daily application at 12-hourly intervals may be necessary. Patients especially newborn, should be closely observed for one to two hours after the first dose and careful monitoring for adverse events should be carried out until surgery is performed.
For a transient treatment in the paediatric population.
Adults, the elderly and children over 12 years
The usual dose is one drop of Timolol 0.25% w/v Eye Drops Solution, in the affected eye(s), twice a day (preferably once in the morning and then once in the evening). However, your doctor may find that you need a stronger dose. You may therefore be told to use one drop of Timolol 0.5% w/v Eye Drops Solution in the affected eye(s), twice a day, instead. Your doctor may also give you another medicinal product to use at the same time as Timolol Eye Drops to help to lower the pressure inside your eyeball.
Your doctor will test the pressure inside your eyeballs, about four weeks after you start using Timolol Eye Drops, to see if it has been reduced enough. If it has, your doctor may tell you to use only one drop a day.
If your doctor has told you to use Timolol Eye Drops to replace another beta-blocker type of eye drop, you should stop using the other type of eye drop at the end of a day (when you have taken all the doses that you should have done in that day) and start using Timolol Eye Drops the next day as your doctor tells you.
If your doctor has told you to use Timolol Eye Drops to replace any eye drops that do not contain a beta-blocker or any tablets you may have been taking for your glaucoma, on the day that your doctor tells you to start using Timolol Eye Drops, use the other product as you normally would, but also use one drop of Timolol 0.25% w/v Eye Drops Solution in the affected eye(s) twice on that day (preferably once in the morning and then once in the evening). Then, the next day, stop using the other medicinal product (eye drops or tablets) completely and continue to use Timolol Eye Drops as directed by your doctor.
Do not allow the tip of the Timolol Eye Drops bottle to touch your eyes or around your eyes, do not touch the tip of the bottle with your fingers, and replace the screw cap tightly onto the bottle every time you finish using Timolol Eye Drops. This will stop Timolol Eye Drops becoming contaminated with bacteria that can cause eye infections which, in turn, can lead to serious eye damage, possibly resulting in loss of vision.
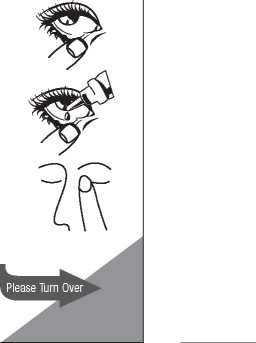
Use Timolol Eye Drops as follows:
1. Before opening a bottle of Timolol Eye Drops for the first time, check that the tamper-evident seal on the cap is not broken - if it is, do not use the timolol contained within it and tell your doctor or pharmacist immediately.
2. To open the bottle, unscrew the cap by turning it anticlockwise.
3. Tilt your head back so you are looking at the ceiling and gently pull the lower eyelid of the affected eye down, so there is a pocket between your eyelid and your eye and hold it there whilst you put the drops in. For paediatric populations, one drop only of Timolol should be instilled per dosing time.
4. Turn the bottle upside down and squeeze to release a drop into your eye. Make sure you do not touch your eye or eye lid or anything else with the dropper tip.
5. After using Timolol Eye Drops, press a finger into the corner of your eye, by the nose, for 2 minutes. This helps to stop timolol maleate getting into the rest of the body. For paediatric populations, after instillation keep the eyes closed for as long as possible (e.g. 3-5 minutes).
6. Repeat steps three and four above for the other eye if you have been told to do so by your doctor.
7. Replace and tighten the bottle cap by turning it clockwise until it is firmly closed, straight away after use. Do not over-tighten the cap.
If you use more Timolol Eye Drops than you should
If you think you have used more Timolol Eye Drops than you should have done, or you accidentally swallow any Timolol Eye Drops, see a doctor or go to the nearest hospital casualty department immediately, taking the bottle of Timolol Eye Drops and this leaflet with you, even if you feel well. Signs that you or somebody else has used too much Timolol Eye Drops can include: dizziness, headache, shortness of breath, a slower than normal heartbeat, difficulty breathing or heart attack (resulting in severe chest pain).
If you forget to use Timolol Eye Drops
If you forget to use Timolol Eye Drops, use it again as soon as you remember and then continue to use as you normally would. However, if it is almost time for your next dose by the time you remember, skip the dose that you have missed completely and wait until it is time for your next dose before you use Timolol Eye Drops again. Do not use a double dose of Timolol Eye Drops to make up for a forgotten dose.
If you stop using Timolol Eye Drops
To be effective Timolol Eye Drops must be used every day - if you stop using Timolol Eye Drops for any reason, contact your doctor or pharmacist immediately.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Timolol Eye Drops can cause side effects, although not everybody gets them.
You can usually carry on taking the drops, unless the effects are serious. If you're worried, talk to a doctor or pharmacist. Do not stop using Timolol Eye Drops without speaking to your doctor.
The frequency of possible side effects listed below is defined using the following convention.
Very common (affects more than 1 user in 10)
Common (affects 1 to 10 users in 100)
Uncommon (affects 1 to 10 users in 1,000)
Rare (affects 1 to 10 users in 10,000)
Not known (frequency cannot be estimated from the available data)
Like other medicines applied into the eyes, timolol is absorbed into the blood. This may cause similar side effects as seen with 'intraveneous' and/or 'oral' as applicable beta-blocking agents. Incidence of side effects after topical opthalmic administration is lower than when medicines are, for example, taken by mouth or injected.
Listed side effects include reactions seen within the class of beta-blockers when used for treating eye conditions. Possible side effects are listed below under the parts of the body that might be affected.
Heart:
• a slower than normal heart rate (bradycardia)
• changes in the rhythm or speed of the heart beat (arrhythmia)
• heart condition (atrioventricular block) (a problem with the electrical conduction system of your heart)
• heart (cardiac) failure (symptoms of which can include shortness of breath)
• palpitations
• heart attack (cardiac arrest) (symptoms of which can include severe chest pain)
• worsening of angina and associated chest pain
• heart disease with shortness of breath and swelling of parts of the body (feet and legs) due to fluid build up (congestive heart failure)
• fluid build up (oedema)
• worsening of pre-existing circulation problems
• cold hands and feet.
Ears:
• ringing noises in the ears
• dizziness.
Eyes:
• eye irritation including a feeling of burning, stinging, itching, tearing, and redness in the eyes
• inflammation of parts of your eyes or eyelids including the cornea making them itchy, red, sticky or swollen
• swollen eyelid (blepharitis)
• irritation or feeling of having something in the eye (keratitis)
• painful eyes or poorer than normal eyesight
• loss of blinking reaction to objects near eye
• drooping of the upper or lower eyelids (ptosis)
• dry eyes
• changes in vision including double vision (diplopia)
• decreased corneal sensitivity
• damage to the front layer of the eyeball (corneal erosion)
• structural damage to the eye (possibly resulting in the appearance of flashes of light or ‘floaters’ in vision, or loss of vision) following eye surgery has also been reported if timolol was being used after the surgery. This includes blurred vision and detachment of the layer below the retina that contains blood vessels, low eye pressure, and visual disturbances after an eye operation (choroidal detachment following filtration surgery).
Digestive system:
• feeling sick
• being sick
• diarrhoea
• indigestion (dyspepsia) including stomach ache, feeling bloated or ‘full’
• dry mouth
• taste disturbances (dysgeusia).
Other parts of your body:
• a lack of strength and weakness generally or when exercising
• Muscle weakness/tiredness (asthenia)
• Muscle pain not caused by exercise (myalgia)
• extreme tiredness (fatigue)
• chest pain
• pain in the fingers and toes.
Immune system:
• generalized allergic reactions including swelling beneath the skin that can occur in areas such as the face, lips, mouth, tongue, throat or limbs, and can obstruct the airway which may cause difficulty swallowing or breathing (angiodema)
• hives (urticaria) or itchy rash
• itchiness (pruritus)
• local or generalised rash
• systemic lupus erythematosus (also known as “SLE" or “Lupus"), symptoms of which can include painful
or swollen joints, muscle pain, unexplained fever or a red rash
• severe sudden life-threatening allergic reaction (anaphylactic reaction).
Metabolism:
• higher than normal blood sugar levels (symptoms of which can include a great thirst, a dry mouth or the need to pass water often)
• lower than normal blood sugar (or glucose) levels (hypoglycaemia) (symptoms of which can include feeling sick, sweating, weakness, faintness, confusion or coma).
Skeleton:
• joint pain.
Nervous system:
• stroke (cerebrovascular accident)
• dizziness
• headache
• fainting (syncope)
• unusual sensations like a feeling of pins and needles (paraesthesia)
• increases in signs and symptoms of, or worsening of a disorder called myasthenia gravis which affects the muscles and causes weakness and tiredness.
Brain:
• depression
• difficulty sleeping (insomnia)
• an increased number of dreams
• nightmares
• memory loss
• lack of concentration.
Reproductive system:
• a reduced sex drive (sexual dysfunction, decreased libido)
• ‘Peyronie’s disease’ in men, causing the penis to bend when erect
• impotence
• difficulty passing water.
Lungs:
• wheezing, difficulty breathing (bronchospasm), particularly if you already have a disease affecting your lungs such as asthma. This may be serious enough to cause death (uaually in patients with pre-existing bronchospastic disease)
• breathlessness (dysponea)
• cough
• fluid in the lungs
• failure of the lungs to function normally (symptoms of which can include bluish skin, confusion or sleepiness).
Skin:
• hair loss (alopecia)
• red, itchy, flaky rash or worsening of pre-existing psoriasis
• itching
• peeling of skin
• purple-coloured spots and patches on the skin
• sweating
• skin rash with white silvery coloured appearance (psoriasiform rash).
Circulatory system:
• abnormally low blood pressure (hypotension)
• a reduced blood supply to the brain (cerebral ischemia) (the symptoms of these can include problems moving, speaking, seeing or thinking clearly)
• pain in your legs on walking
• redness and flushing caused by increased blood flow to the skin
• poor blood circulation - Raynaud’s phenomenon (which causes the fingers and/or toes to turn white, blue or red in hot or cold temperatures).
If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. HOW TO STORE TIMOLOL EYE DROPS
Keep out of the reach and sight of children.
Do not use Timolol Eye Drops after the expiry date which is stated on the label and the outer carton. The expiry date refers to the last day of that month.
Do not store above 25°C.
Keep the bottle in the original outer cardboard carton in order to protect from light.
You can use Timolol Eye Drops for only four weeks after first opening the bottle, to prevent infections. Discard the opened bottle with any remaining solution after that time.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What Timolol Eye Drops contain
The active substance is timolol. One ml of Timolol 0.25% w/v Eye Drops solution contains 2.5 mg of timolol (as 3.4mg of timolol maleate). One ml of Timolol 0.5% w/v Eye Drops Solution contains 5 mg of timolol (as 6.8mg of timolol maleate).
The other ingredients are disodium hydrogen phosphate dodecahydrate, sodium dihydrogen phosphate dihydrate, sodium chloride, benzalkonium chloride, sodium hydroxide (E524) (0.08% w/v in timolol 0.25% w/v eye drops, solution and 0.1% w/v in timolol 0.5% w/v eye drops, solution) and water for injections.
What Timolol Eye Drops looks like and contents of the pack
Timolol is a clear, colourless to light yellow, solution that is used as an eye drop.
Timolol Eye Drops are available in a 5 ml natural plastic (low density polyethylene) bottle, containing 5 ml of timolol eye drop solution, fitted with a natural plastic (low density polyethylene) plug and a white plastic (high density polyethylene) screw cap with a tamper-evident seal, contained within a cardboard carton.
Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder:
Wockhardt UK Ltd, Ash Road North, Wrexham, LL13 9UF, U.K.
Manufacturer:
CP Pharmaceuticals Ltd, Ash Road North, Wrexham, LL13 9UF, U.K.
Other formats:
To listen to or request a copy of this leaflet in Braille, large print or audio please call, free of charge:
0800 198 5000 (UK Only)
Please be ready to give the following information:
|
Product name |
Reference number |
|
Timolol 0.25% w/v Eye Drops, Solution |
29831/0353 |
|
Timolol 0.5% w/v Eye Drops, Solution |
29831/0354 |
This is a service provided by the Royal National Institute of Blind People.
For the Republic of Ireland please call +353 52 6186000.
These medicinal products are authorised in the following Member States in the EEA, under the following names:
Cyprus - Timolol Wockhardt 0.25% w/v Eye Drops, Solution; Timolol Wockhardt 0.5% w/v Eye Drops, Solution
Germany - Timolol 0.25% w/v Eye Drops, Solution; Timolol 0.5% w/v Eye Drops, Solution
Malta - Timolol 0.25% w/v Eye Drops, Solution; Timolol 0.5% w/v Eye Drops, Solution
Republic of Ireland - Timolol 0.25% w/v Eye Drops, Solution; Timolol 0.5% w/v Eye Drops, Solution
United Kingdom - Timolol 0.25% w/v Eye Drops, Solution; Timolol 0.5% w/v Eye Drops, Solution
This leaflet was last approved in December 2011.

pg2/2