Timoptol 0.25% W/V Unit Dose Eye Drops Solution
S1550 LEAFLET Timoptol 20151130
PACKAGE LEAFLET: INFORMATION FOR THE USER TIMOPTOL 0.25% w/v UNIT DOSE EYE DROPS SOLUTION (timolol as maleate)
Your medicine is known as Timoptol 0.25% Unit Dose Ophthalmic Solution but will be referred to as Timoptol Unit Dose throughout the following patient information leaflet.
Information for other strength of Timoptol Unit Dose also may be present in this leaflet.
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Timoptol Unit Dose is and what it is used for
2. Before you use Timoptol Unit Dose
3. How to use Timoptol Unit Dose
4. Possible side effects
5. How to store Timoptol Unit Dose
6. Further information
1. WHAT TIMOPTOL UNIT DOSE IS AND WHAT IT IS USED FOR
Timoptol Unit Dose contains a substance called timolol which belongs to a group of medicines called beta-blockers. Timolol lowers the pressure in your eye(s). It is used to treat glaucoma, when the pressure in the eye is raised.
2. BEFORE YOU USE TIMOPTOL UNIT DOSE
Do not use Timoptol Unit Dose if:
• you are allergic (hypersensitive) to timolol, beta-blockers or any of the other ingredients of Timoptol Unit Dose (see section 6 for Further Information)
• you have now or have had in the past respiratory problems such as asthma, severe chronic obstructive bronchitis (severe lung disease which may cause wheeziness, difficulty in breathing and/or long-standing cough)
• you have heart problems
• slow heart beat
• disorders of heart rhythm (irregular heartbeats)
• heart failure
- “cardiogenic shock” - a serious heart condition caused by very low blood pressure, which may result in the following symptoms: dizziness and lightheadedness, fast pulse rate, white skin, sweating, restlessness, loss of consciousness.
If you are not sure whether you should use Timoptol Unit Dose talk to your doctor or pharmacist.
Take special care with Timoptol Unit Dose
Before you use this medicine tell your doctor if you have now or
have had in the past
• coronary heart disease (symptoms can include chest pain or tightness, breathlessness or choking), heart failure,
• low blood pressure
• disturbances of heart rate such as slow heartbeat
• breathing problems, asthma or chronic obstructive pulmonary disease
• poor blood circulation disease (such as Raynaud's disease or Raynaud's syndrome)
• diabetes as timolol may mask signs and symptoms of low blood sugar
• overactivity of the thyroid gland as timolol maleate may mask signs and symptoms
• you wear soft contact lenses.
Tell your doctor before you have an operation that you are using Timoptol Unit Dose as timolol may change effects of some medicines used during anaesthesia.
If your eye becomes irritated or any new eye problems come on, talk to your doctor straight away. Eye problems could include redness of the eye or swelling of the eyelids (see Section 4:
Possible Side Effects).
If you suspect that Timoptol Unit Dose is causing an allergic reaction or hypersensitivity (for example, skin rash, or redness and itching of the eye), stop using Timoptol Unit Dose and contact your doctor immediately.
Tell your doctor if:
• you get an eye infection
• you injure your eye or have an operation on it
• your eye problems get worse or you get any new symptoms.
Use in children and adolescents
There is only very limited data available on the use of timolol in infants and children. For example, in one small clinical study, timolol, the active ingredient in Timoptol Unit Dose eye drops, has been studied in infants and children aged 12 days to 5 years, who have raised pressure in the eye(s) or have been diagnosed with glaucoma. For more information, talk to your doctor.
Timoptol Unit Dose should generally be used with caution in young patients. In newborns, infants and younger children Timoptol Unit Dose should be used with extreme caution.
If coughing, wheezing, abnormal breathing or abnormal pauses in breathing (apnoea) occur, the use of the medication should be stopped immediately. Contact your doctor as soon as possible.
A portable apnoea monitor may also be helpful.
Using other medicines
Timoptol Unit Dose can affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Tell your doctor if you are using or intend to use medicines to lower blood pressure, heart medicine or medicines to treat diabetes. Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including other eye drops or medicines obtained without a prescription. It is important to tell your doctor before using Timoptol Unit Dose if you are taking one or more of the following medicines:
• a calcium antagonist, such as nifedipine, verapamil or diltiazem, often used to treat high blood pressure, angina, an abnormal heartbeat or Raynaud's syndrome
• digoxin, a medicine used to relieve heart failure or treat abnormal heartbeat
• medicines known as catecholamine-depleting agents, such as rauwolfia alkaloids/reserpine used for high blood pressure
• medicines called pressor amines, such as adrenaline used to treat severe allergic reaction
• quinidine (used to treat heart conditions and some types of malaria)
• antidepressants known as fluoxetine and paroxetine
• clonidine, a medicine used to treat high blood pressure
• other beta-blockers taken by mouth or used as eye drops, because they belong to the same group of medicines as Timoptol Unit Dose and could have an additive effect.
Pregnancy and breast-feeding
Ask your doctor for advice before taking any medicine.
Use in pregnancy
Do not use Timoptol Unit Dose if you are pregnant unless your doctor considers it necessary.
Use in breast-feeding
Do not use Timoptol Unit Dose if you are breast-feeding. Timolol may get into your milk. Ask your doctor for advice before taking any medicine during breast-feeding
Driving and using machines
There are possible side effects associated with Timoptol Unit Dose, such as dizziness; tiredness and changes in your eyesight such as blurred vision, drooping of the upper eyelid (making the eye stay half closed), double vision; which may affect your ability to drive and/or operate machinery. Do not drive and/or operate machinery until you feel well and your vision is clear.
3. HOW TO USE TIMOPTOL UNIT DOSE
Always use Timoptol Unit Dose exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure. The doctor will decide how many drops you should take each day and how long you should use them.
The usual dose is one drop in the affected eye(s) twice each day:
• one in the morning
• one in the evening.
Do not change the dose of this medicine without talking to your doctor.
Do not allow the tip of the single dose container to touch the eye or areas around the eye. It may become contaminated with bacteria that can cause eye infection leading to serious damage of the eye, even loss of vision. To avoid possible contamination of the single dose container, keep the tip of the single dose container away from contact with any surface.
Instructions for use.
Open the drops container just before you want to use it. After using the drops, throw away what is left. This is because the drops cannot be kept free of bacteria after being opened.
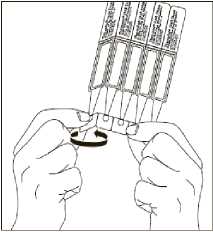
1. Open the foil sachet containing the individual single dose containers. There are three strips of 5 single dose containers in each sachet.
2. First wash your hands and then break off a single dose container from the strip. Then twist open the top of the single dose container as shown.
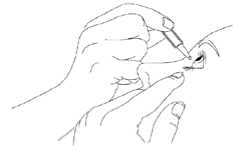
3. Tilt your head back and pull your lower eyelid now slightly.
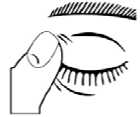
5. After using Timoptol Unit Dose, press a finger into the corner of your eye, by the nose for 2 minutes. This helps to stop timolol getting into the rest of your body.
6. After putting the drop into the eye(s), throw away the used container, even if there is solution remaining, to avoid contamination.
7. Store the remaining containers in the foil sachet. The containers must be used within 15 days after opening the sachet. If any containers are left 15 days after opening the sachet, throw them away and open a new one.
Children and Adolescents
Before you or your child starts to take Timoptol Unit Dose, your doctor or your child's doctor will have carried out a detailed medical examination and decided whether or not this medicine is suitable. You or your child, especially a newborn, should be closely monitored for one to two hours after the first dose and carefully monitored for any signs of side effects until surgery is carried out. Method of administration:
One drop only of Timoptol Unit Dose should be instilled into the affected eye(s) each time. Follow the “Instructions for Use” above when administering the eye drops. After instillation keep the eyes closed for as long as possible (e.g. 3 - 5 minutes) and apply pressure to the corner of the eye closest to the nose to prevent the eye drops spreading throughout the body.
Duration of treatment:
Your doctor or your child's doctor will decide for how long the eye drops will be needed.
If you use more Timoptol Unit Dose than you should
If you put too many drops in your eye or swallow any of the drops, you may:
• have a headache
• feeling dizzy or light-headed
• have difficulty breathing
• chest pain
• feel that your heart rate has slowed down.
If this happens, contact your doctor immediately.
If you forget to use Timoptol Unit Dose
It is important to take Timoptol Unit Dose as prescribed by your
doctor.
• If you miss a dose, use the drops as soon as possible.
• If it is almost time for the next dose, skip the missed dose and take the next dose at the usual time.
• Do not take a double dose to make up for the forgotten dose.
If you stop using Timoptol Unit Dose
If you want to stop using this medicine talk to your doctor first.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines Timoptol Unit Dose can cause side effects, although not everybody gets them.
You can usually carry on taking the drops, unless the effects are serious. If you're worried, talk to a doctor or pharmacist. Do not stop using Timoptol Unit Dose without speaking to your doctor.
Like other medicines applied into eyes, timolol is absorbed into the blood. This may cause similar side effects as seen with intravenous and/or oral beta-blocking agents. Incidence of side effects after topical ophthalmic administration is lower than when medicines are, for example taken by mouth or injected. Listed side effects include reactions seen within the class of beta-blockers when used for treating eye conditions.
If you develop allergic reactions including swelling beneath the skin that can occur in areas such as the face and limbs, and can obstruct the airway which may cause difficulty swallowing or breathing, hives or itchy rash, localized and generalized rash, itchiness, severe sudden life-threatening allergic reaction, stop taking your eye drops and seek medical attention immediately.
Low blood glucose levels.
Difficulty sleeping (insomnia), depression, nightmares, memory loss.
Fainting, stroke, reduced blood supply to the brain, increases in signs and symptoms of myasthenia gravis (muscle disorder), dizziness, unusual sensations like tingling or pins and needles, and headache.
Signs and symptoms of eye irritation (e.g. burning, stinging, itching, tearing, redness), inflammation of the eyelid, inflammation in the cornea, blurred vision and detachment of the layer below the retina that contains blood vessels following filtration surgery which may cause visual disturbances, decreased corneal sensitivity, dry eyes, corneal erosion (damage to the front layer of the eyeball), drooping of the upper eyelid (making the eye stay half closed) double vision, sensitivity to light, discharge from the eye, pain in the eye.
Ringing sound in the ears.
Slow heart rate, chest pain, palpitations, oedema (fluid build up), changes in the rhythm or speed of the heartbeat, congestive heart failure (heart disease with shortness of breath and swelling of the feet and legs due to fluid build up), a type of heart rhythm disorder, heart attack, heart failure.
Low blood pressure, fainting, interference with the blood supply to the brain which may lead to a stroke, Raynaud's phenomenon, cold hands and feet, limping because there is a reduced blood supply to your legs.
Constriction of the airways in the lungs (predominantly in patients with pre-existing disease), difficulty breathing, shortness of breath, wheezing, cough.
Taste disturbances, nausea, indigestion, diarrhoea, dry mouth, abdominal pain, vomiting.
Sexual dysfunction, decreased sex drive, decreased libido. In men a condition which affects your penis called Peyronie's disease. The signs may be abnormal curve, pain or hardening of the tissue of your penis.
Hair loss, skin rash with white silvery coloured appearance (psoriasiform rash) or worsening of psoriasis, skin rash, itching. Muscle weakness/tiredness, muscle pain not caused by exercise.
A condition called lupus (systemic lupus erythematosus).
Ask your doctor or pharmacist for more information about the side effects. Both have a more complete list of side effects.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE TIMOPTOL UNIT DOSE
• Keep out of the sight and reach of children.
• Store in the original package to protect from light.
• Do not store your drops above 25°C.
• Do not use Timoptol Unit Dose after the expiry date which is stated on the label and carton. The expiry date refers to the last day of the month.
• If the medicine becomes discoloured or shows any other signs of deterioration, you should seek the advice of your pharmacist who will tell you what to do.
• If you have any unwanted Timoptol Unit Dose, don't dispose of them in waste water or household rubbish. Take them back to your pharmacist, who will dispose of them in a way that won't harm the environment
6. FURTHER INFORMATION
What Timoptol Unit Dose contains
• Each ml contains timolol maleate equivalent to 2.5mg timolol as the active ingredient.
• Also contains sodium hydroxide, water for injection, disodium phosphate dodecahydrate and sodium dihydrogen phosphate dihydrate.
What Timoptol Unit Dose looks like and contents of the pack
Timoptol Unit Dose is available in cartons containing 30 unit doses.
Each ‘Timoptol' Unit dose dispenser contains 0.2ml clear colourless liquid without preservative.
Product Licence holder
Procured from within the EU and repackaged by the Product Licence holder: S&M Medical Ltd, Chemilines House,
Wembley, HA0 1DX.
Manufacturer
This product is manufactured by Laboratoires Merck Sharp & Dohme-Chibret, Riom 63963, Clermont-Ferrand Cedex 9, France.
| POM | PL: 19488/1550
Leaflet revision date: 30 November 2015
Timoptol is a registered trade mark of Merck & Co Inc, USA.
This leaflet gives the most important patient information about Timoptol Unit Dose. If you have any questions after you have read it, ask your doctor or pharmacist who will give you further information.
Further information about glaucoma is available from:
International Glaucoma Association (IGA)
15A Highpoint Business Village
Henwood, Ashford
Kent, TN24 8DH
Tel: 01233 648170
E-mail: info@iga.org.uk
Registered Charity number 274681.
(The IGA is an independent charity organisation which helps glaucoma patients and their relatives, and is not associated with Santen Oy.)
Alternatively, if you or someone you know has problems with their vision, and you require further advice or information, please phone the Royal National Institute for the Blind (RNIB) Helpline on 0845 776 9999, Monday to Friday 9am to 5 pm, calls charged at local rates.
(The RNIB is an independent UK charity and is not associated with Santen Oy).
S1550 LEAFLET Timoptol 20151130
S1550 LEAFLET Timolol 20151130
PACKAGE LEAFLET: INFORMATION FOR THE USER TIMOLOL 0.25% w/v UNIT DOSE EYE DROPS SOLUTION (timolol as maleate)
Your medicine is known as Timolol 0.25% Unit Dose Ophthalmic Solution but will be referred to as Timolol Unit Dose throughout the following patient information leaflet.
Information for other strength of Timolol Unit Dose also may be present in this leaflet.
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Timolol Unit Dose is and what it is used for
2. Before you use Timolol Unit Dose
3. How to use Timolol Unit Dose
4. Possible side effects
5. How to store Timolol Unit Dose
6. Further information
1. WHAT TIMOLOL UNIT DOSE IS AND WHAT IT IS USED FOR
Timolol Unit Dose contains a substance called timolol which belongs to a group of medicines called beta-blockers. Timolol lowers the pressure in your eye(s). It is used to treat glaucoma, when the pressure in the eye is raised.
2. BEFORE YOU USE TIMOLOL UNIT DOSE
Do not use Timolol Unit Dose if:
• you are allergic (hypersensitive) to timolol, beta-blockers or any of the other ingredients of Timolol Unit Dose (see section 6 for Further Information)
• you have now or have had in the past respiratory problems such as asthma, severe chronic obstructive bronchitis (severe lung disease which may cause wheeziness, difficulty in breathing and/or long-standing cough)
• you have heart problems
• slow heart beat
• disorders of heart rhythm (irregular heartbeats)
• heart failure
- “cardiogenic shock” - a serious heart condition caused by very low blood pressure, which may result in the following symptoms: dizziness and lightheadedness, fast pulse rate, white skin, sweating, restlessness, loss of consciousness.
If you are not sure whether you should use Timolol Unit Dose talk to your doctor or pharmacist.
Take special care with Timolol Unit Dose
Before you use this medicine tell your doctor if you have now or
have had in the past
• coronary heart disease (symptoms can include chest pain or tightness, breathlessness or choking), heart failure,
• low blood pressure
• disturbances of heart rate such as slow heartbeat
• breathing problems, asthma or chronic obstructive pulmonary disease
• poor blood circulation disease (such as Raynaud's disease or Raynaud's syndrome)
• diabetes as timolol may mask signs and symptoms of low blood sugar
• overactivity of the thyroid gland as timolol maleate may mask signs and symptoms
• you wear soft contact lenses.
Tell your doctor before you have an operation that you are using Timolol Unit Dose as timolol may change effects of some medicines used during anaesthesia.
If your eye becomes irritated or any new eye problems come on, talk to your doctor straight away. Eye problems could include redness of the eye or swelling of the eyelids (see Section 4:
Possible Side Effects).
If you suspect that Timolol Unit Dose is causing an allergic reaction or hypersensitivity (for example, skin rash, or redness and itching of the eye), stop using Timolol Unit Dose and contact your doctor immediately.
Tell your doctor if:
• you get an eye infection
• you injure your eye or have an operation on it
• your eye problems get worse or you get any new symptoms.
Use in children and adolescents
There is only very limited data available on the use of timolol in infants and children. For example, in one small clinical study, timolol, the active ingredient in Timolol Unit Dose eye drops, has been studied in infants and children aged 12 days to 5 years, who have raised pressure in the eye(s) or have been diagnosed with glaucoma. For more information, talk to your doctor.
Timolol Unit Dose should generally be used with caution in young patients. In newborns, infants and younger children Timolol Unit Dose should be used with extreme caution.
If coughing, wheezing, abnormal breathing or abnormal pauses in breathing (apnoea) occur, the use of the medication should be stopped immediately. Contact your doctor as soon as possible.
A portable apnoea monitor may also be helpful.
Using other medicines
Timolol Unit Dose can affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Tell your doctor if you are using or intend to use medicines to lower blood pressure, heart medicine or medicines to treat diabetes. Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including other eye drops or medicines obtained without a prescription. It is important to tell your doctor before using Timolol Unit Dose if you are taking one or more of the following medicines:
• a calcium antagonist, such as nifedipine, verapamil or diltiazem, often used to treat high blood pressure, angina, an abnormal heartbeat or Raynaud's syndrome
• digoxin, a medicine used to relieve heart failure or treat abnormal heartbeat
• medicines known as catecholamine-depleting agents, such as rauwolfia alkaloids/reserpine used for high blood pressure
• medicines called pressor amines, such as adrenaline used to treat severe allergic reaction
• quinidine (used to treat heart conditions and some types of malaria)
• antidepressants known as fluoxetine and paroxetine
• clonidine, a medicine used to treat high blood pressure
• other beta-blockers taken by mouth or used as eye drops, because they belong to the same group of medicines as Timolol Unit Dose and could have an additive effect.
Pregnancy and breast-feeding
Ask your doctor for advice before taking any medicine.
Use in pregnancy
Do not use Timolol Unit Dose if you are pregnant unless your doctor considers it necessary.
Use in breast-feeding
Do not use Timolol Unit Dose if you are breast-feeding. Timolol may get into your milk. Ask your doctor for advice before taking any medicine during breast-feeding
Driving and using machines
There are possible side effects associated with Timolol Unit Dose, such as dizziness; tiredness and changes in your eyesight such as blurred vision, drooping of the upper eyelid (making the eye stay half closed), double vision; which may affect your ability to drive and/or operate machinery. Do not drive and/or operate machinery until you feel well and your vision is clear.
3. HOW TO USE TIMOLOL UNIT DOSE
Always use Timolol Unit Dose exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure. The doctor will decide how many drops you should take each day and how long you should use them.
The usual dose is one drop in the affected eye(s) twice each day:
• one in the morning
• one in the evening.
Do not change the dose of this medicine without talking to your doctor.
Do not allow the tip of the single dose container to touch the eye or areas around the eye. It may become contaminated with bacteria that can cause eye infection leading to serious damage of the eye, even loss of vision. To avoid possible contamination of the single dose container, keep the tip of the single dose container away from contact with any surface.
Instructions for use.
Open the drops container just before you want to use it. After using the drops, throw away what is left. This is because the drops cannot be kept free of bacteria after being opened.
1. Open the foil sachet containing the individual single dose containers. There are three strips of 5 single dose containers in each sachet.
2. First wash your hands and then break off a single dose container from the strip. Then twist open the top of the single dose container as shown.
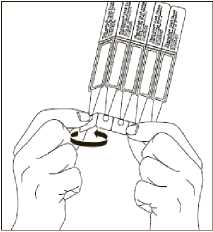
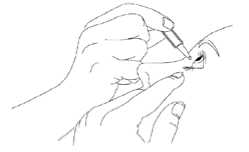
3. Tilt your head back and pull your lower eyelid now slightly.
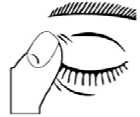
5. After using Timolol Unit Dose, press a finger into the corner of your eye, by the nose for 2 minutes. This helps to stop timolol getting into the rest of your body.
6. After putting the drop into the eye(s), throw away the used container, even if there is solution remaining, to avoid contamination.
7. Store the remaining containers in the foil sachet. The containers must be used within 15 days after opening the sachet. If any containers are left 15 days after opening the sachet, throw them away and open a new one.
Children and Adolescents
Before you or your child starts to take Timolol Unit Dose, your doctor or your child's doctor will have carried out a detailed medical examination and decided whether or not this medicine is suitable. You or your child, especially a newborn, should be closely monitored for one to two hours after the first dose and carefully monitored for any signs of side effects until surgery is carried out. Method of administration:
One drop only of Timolol Unit Dose should be instilled into the affected eye(s) each time. Follow the “Instructions for Use” above when administering the eye drops. After instillation keep the eyes closed for as long as possible (e.g. 3 - 5 minutes) and apply pressure to the corner of the eye closest to the nose to prevent the eye drops spreading throughout the body.
Duration of treatment:
Your doctor or your child's doctor will decide for how long the eye drops will be needed.
If you use more Timolol Unit Dose than you should
If you put too many drops in your eye or swallow any of the drops, you may:
• have a headache
• feeling dizzy or light-headed
• have difficulty breathing
• chest pain
• feel that your heart rate has slowed down.
If this happens, contact your doctor immediately.
If you forget to use Timolol Unit Dose
It is important to take Timolol Unit Dose as prescribed by your
doctor.
• If you miss a dose, use the drops as soon as possible.
• If it is almost time for the next dose, skip the missed dose and take the next dose at the usual time.
• Do not take a double dose to make up for the forgotten dose.
If you stop using Timolol Unit Dose
If you want to stop using this medicine talk to your doctor first.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines Timolol Unit Dose can cause side effects, although not everybody gets them.
You can usually carry on taking the drops, unless the effects are serious. If you're worried, talk to a doctor or pharmacist. Do not stop using Timolol Unit Dose without speaking to your doctor.
Like other medicines applied into eyes, timolol is absorbed into the blood. This may cause similar side effects as seen with intravenous and/or oral beta-blocking agents. Incidence of side effects after topical ophthalmic administration is lower than when medicines are, for example taken by mouth or injected. Listed side effects include reactions seen within the class of beta-blockers when used for treating eye conditions.
If you develop allergic reactions including swelling beneath the skin that can occur in areas such as the face and limbs, and can obstruct the airway which may cause difficulty swallowing or breathing, hives or itchy rash, localized and generalized rash, itchiness, severe sudden life-threatening allergic reaction, stop taking your eye drops and seek medical attention immediately.
Low blood glucose levels.
Difficulty sleeping (insomnia), depression, nightmares, memory loss.
Fainting, stroke, reduced blood supply to the brain, increases in signs and symptoms of myasthenia gravis (muscle disorder), dizziness, unusual sensations like tingling or pins and needles, and headache.
Signs and symptoms of eye irritation (e.g. burning, stinging, itching, tearing, redness), inflammation of the eyelid, inflammation in the cornea, blurred vision and detachment of the layer below the retina that contains blood vessels following filtration surgery which may cause visual disturbances, decreased corneal sensitivity, dry eyes, corneal erosion (damage to the front layer of the eyeball), drooping of the upper eyelid (making the eye stay half closed) double vision, sensitivity to light, discharge from the eye, pain in the eye.
Ringing sound in the ears.
Slow heart rate, chest pain, palpitations, oedema (fluid build up), changes in the rhythm or speed of the heartbeat, congestive heart failure (heart disease with shortness of breath and swelling of the feet and legs due to fluid build up), a type of heart rhythm disorder, heart attack, heart failure.
Low blood pressure, fainting, interference with the blood supply to the brain which may lead to a stroke, Raynaud's phenomenon, cold hands and feet, limping because there is a reduced blood supply to your legs.
Constriction of the airways in the lungs (predominantly in patients with pre-existing disease), difficulty breathing, shortness of breath, wheezing, cough.
Taste disturbances, nausea, indigestion, diarrhoea, dry mouth, abdominal pain, vomiting.
Sexual dysfunction, decreased sex drive, decreased libido. In men a condition which affects your penis called Peyronie's disease. The signs may be abnormal curve, pain or hardening of the tissue of your penis.
Hair loss, skin rash with white silvery coloured appearance (psoriasiform rash) or worsening of psoriasis, skin rash, itching. Muscle weakness/tiredness, muscle pain not caused by exercise.
A condition called lupus (systemic lupus erythematosus).
Ask your doctor or pharmacist for more information about the side effects. Both have a more complete list of side effects.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE TIMOLOL UNIT DOSE
• Keep out of the sight and reach of children.
• Store in the original package to protect from light.
• Do not store your drops above 25°C.
• Do not use Timolol Unit Dose after the expiry date which is stated on the label and carton. The expiry date refers to the last day of the month.
• If the medicine becomes discoloured or shows any other signs of deterioration, you should seek the advice of your pharmacist who will tell you what to do.
• If you have any unwanted Timolol Unit Dose, don't dispose of them in waste water or household rubbish. Take them back to your pharmacist, who will dispose of them in a way that won't harm the environment
6. FURTHER INFORMATION
What Timolol Unit Dose contains
• Each ml contains timolol maleate equivalent to 2.5mg timolol as the active ingredient.
• Also contains sodium hydroxide, water for injection, disodium phosphate dodecahydrate and sodium dihydrogen phosphate dihydrate.
What Timolol Unit Dose looks like and contents of the pack
Timolol Unit Dose is available in cartons containing 30 unit doses.
Each ‘Timolol' Unit dose dispenser contains 0.2ml clear colourless liquid without preservative.
Product Licence holder
Procured from within the EU and repackaged by the Product Licence holder: S&M Medical Ltd, Chemilines House,
Wembley, HA0 1DX.
Manufacturer
This product is manufactured by Laboratoires Merck Sharp & Dohme-Chibret, Riom 63963, Clermont-Ferrand Cedex 9, France.
| POM | PL: 19488/1550
Leaflet revision date: 30 November 2015
This leaflet gives the most important patient information about Timolol Unit Dose. If you have any questions after you have read it, ask your doctor or pharmacist who will give you further information.
Further information about glaucoma is available from:
International Glaucoma Association (IGA)
15A Highpoint Business Village
Henwood, Ashford
Kent, TN24 8DH
Tel: 01233 648170
E-mail: info@iga.org.uk
Registered Charity number 274681.
(The IGA is an independent charity organisation which helps glaucoma patients and their relatives, and is not associated with Santen Oy.)
Alternatively, if you or someone you know has problems with their vision, and you require further advice or information, please phone the Royal National Institute for the Blind (RNIB) Helpline on 0845 776 9999, Monday to Friday 9am to 5 pm, calls charged at local rates.
(The RNIB is an independent UK charity and is not associated with Santen Oy).
S1550 LEAFLET Timolol 20151130