Tinzaparin Sodium Syringe 10 000 Iu/Ml

Please return to:
LEO Pharma A/S Internal Market Access Industriparken 55 - DK-2750 - Ballerup
|
Scale |
Get-up |
Item No. |
Rev. No. |
Sent by e-mail | |
|
100% |
GB |
031279 |
XX |
T | |
|
Subject INS 410 X 315 mm (205 x 315 mm with 2 foils) |
Date 16/02/15 |
Date |
Date | ||
|
Colour Black |
Sign. JUG |
Sign. |
Sign. | ||
Preparation
Strength
11
Page 1 of 2 font 9 pt and lines space 3,35 mm_Only for 10 syringes Mock-up for registration purpose.
innohep® syringe 10,000 IU/ml
Supplier / Place of production
France
031279-XX

185 mm

|
2 PROOF jug | ||||
|
Date 17/02/15 |
Graphic Design |
Editorial Proof |
Second Approver | |
|
New proof |
According to: |
According to: |
Product name |
□ |
|
requested □ |
Instruction For Use, Mock-up Approval Stamp (MAS) SOP 000647 and |
Instruction For Use, Mock-up Approval Stamp (MAS) SOP 000647 and |
Dosage form |
□ |
|
Sign.: |
Strength/Stripes |
□ | ||
|
SOP_000962 □ |
SOP_000962 □ |
Pack size |
□ | |
|
Sign.: Date: |
Sign.: Date: |
Prompts |
□ | |
|
Date: |
Item No./Reg. No. |
□ | ||
|
Barcode |
□ | |||
|
Sign.: |
Date: | |||
Package leaflet: Information for the user
innohep® syringe 10,000 lU/ml
tinzaparin sodium
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
• In this leaflet innohep 10,000 lU/ml syringe will be called innohep.
What is in this leaflet
1. What innohep® is and what it is used for
2. What you need to know before you use innohep®
3. Howto use innohep®
4. Possible side effects
5. Howto store innohep®
6. Contents of pack and other information
1. What innohep® is and what it is used for
nnohep is a type of heparin - a low molecular weight heparin -and belongs to a group of medicines called anticoagulants; these medicines affect how the blood clots, nnohep prevents clotting, allowing normal blood flowthrough the arteries and veins.
innohep is used in adults to help stop:
Harmful blood clots forming in your veins following an operation. An example is a clot in a deep vein (deep vein thrombosis, DVT). This usually occurs in a leg.
Harmful blood clots forming in the tubing of an artificial kidney machine during kidney dialysis (haemodialysis).
2. What you need to know before you use innohep®
Do not use innohep
If you are allergic (hypersensitive) to tinzaparin or any of the other ingredients of this medicine; you can find a list of ingredients in section 6 of this leaflet.
If you have ever had a reaction to heparin that caused a severe drop in the number of your clotting cells (platelets) -this reaction is called heparin-induced thrombocytopenia (HIT). If you have ever had a major bleed (for instance: in the brain, spine, eye or stomach, into a muscle or the womb, or any conditions which make you bleed severely, such as haemophilia).
If you have a condition called septic endocarditis (an inflammation of the lining of the heart and heart valves).
Warnings and precautions
Important: If you are having an epidural/spinal anaesthetic
Your doctor will decide if you can have an epidural/spinal anaesthetic if you are using innohep.
You must wait 12 hours after your last injection of innohep before having a lumbar puncture or epidural/spinal anaesthetic placed.
You must wait at least 4 hours after having a spinal anaesthetic, or after the catheter has been removed, before you start using innohep again.
If you have an anaesthetic your doctor or nurse will make regular checks. This is to check if you are getting any major bleeding or bruising around your spine. This may cause paralysis that could be permanent. Any signs this may be happening to you include tingling, weakness or numbness in your lower legs or body, back pain or problems in going to the toilet. This happens very rarely.
You may have a blood test before you start using this medicine and at intervals while you are using it; this is to check the level of the clotting cells (platelets) and potassium in your blood.
Do not inject innohep into a muscle. See section 3, "Howto use innohep®".
This medicine may make you bleed more easily, so when you are being given other injections or having any procedures carried out, tell the doctor, nurse or dentist that you are using innohep.
Talk to your doctor, pharmacist or nurse before using innohep
• If you are pregnant, or think you may be pregnant. See the section "Pregnancy and breast-feeding".
• If you have a condition which makes you more likely to bleed.
• If you are being treated with any other injections into your muscles.
• If you are allergic (hypersensitive) to heparin.
• If you are allergic to other low molecular weight heparins, such as enoxaparin or dalteparin.
• If you have any medical condition such as diabetes mellitus or metabolic acidosis which may cause high levels of potassium in your blood (hyperkalaemia).
• If you have an artificial heart valve.
• If you have kidney problems.
innohep should not be interchanged with other low molecular weight heparin products. This is because they are not exactly the same and you could experience problems with your blood clotting.
Elderly people
Because kidney problems are more likely if you are elderly, you may have a blood test to check how well your kidneys are working and to monitor the activity of innohep.
Children and adolescents
innohep is not intended for use in children and adolescents under the age of 18 years.
Other medicines and innohep
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. This includes any medicines bought without a prescription.
You must tell your doctor or pharmacist if you are taking any of the following medicines as you may be likely to bleed more easily:
• Non-steroidal anti-inflammatory drugs (such as ibuprofen or diclofenac): for arthritis or aches or pains.
• Aspirin: either for reducing pain and inflammation, or the lower dose for thinning of the blood.
• Platelet aggregation inhibitors (such as clopidogrel): for stopping harmful blood clots forming.
• Thrombolytic agents (such as streptokinase): for dissolving blood clots.
• Vitamin K antagonists (such as warfarin): for stopping harmful blood clots.
• Activated protein C: for getting rid of blood clots.
• Anticoagulation, taken by mouth (such as rivaroxaban, dabigatran or apixaban): for stopping harmful blood clots.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor, pharmacist or midwife for advice before using this medicine.
Special precautions are required if you will have an epidural anaesthetic to help you with your labour when you are using innohep. (See "Warnings and Precautions").
Driving and using machines
This medicine should not have any effect on your ability to drive or use machines. However, you should check with your doctor if you feel any side effect that may stop you from driving or using machines.
Important information about some of the ingredients of innohep
innohep syringe contains:
• Sodium. This medicine is nearly "sodium free".
Your medicine contains less than 23 milligrams (mg) of sodium per dose.
Please ask your doctor or pharmacist if you are worried about any of the ingredients in this medicine.

|
Scale |
Get-up |
Item No. |
Rev. No. |
Sent by e-mail | |
|
100% |
GB |
031279 |
XX |
T | |
|
Subject INS 410 X 315 mm (205 x 315 mm with 2 foils) |
Date 16/02/15 |
Date |
Date | ||
|
Colour Black |
Sign. JUG |
Sign. |
Sign. | ||
Please return to:
LEO Pharma A/S Internal Market Access Industriparken 55 - DK-2750 - Ballerup
|
Preparation |
Supplier / Place of production |
|
strength fmQfoep® syringe 10,000 IU/ml |
France |
|
Comments: | |
|
Page 2 of 2 |
3. How to use innohep®
Your doctor may decide that you or a carer may inject this medicine. You will be shown how to do the injection and should only do the injection when you have been instructed how to do so.
Always use this medicine exactly as your doctor, pharmacist or nurse has told you. Check with one of them if you are not sure that you understand how to do the injection or if you are unsure about anything else to do with the medicine.
How much innohep to use
Adults, including the elderly:
To prevent harmful blood clots forming in the tubing of an artificial kidney machine during kidney dialysis (haemodialysis)
innohep will be given either into the artificial kidney machine or into your vein.
The usual dose depends on the length of dialysis.
To prevent harmful blood clots forming in your veins after an operation
The usual dose depends on the type of operation you are having. You will have one dose of innohep before your operation.
Then usually you will have one dose of innohep once a day for 7 to 10 days afterwards. Your doctor will decide how long you should use innohep.
Use in children and adolescents
There is limited experience of use in children and adolescents, innohep is not intended for use in children and adolescents under the age of 18 years.
How to inject yourself with innohep
You should inject yourself exactly as you have been shown and only on the parts of your body that you have been told it is safe to inject into. The type of injection you will be giving is known as a subcutaneous injection. The injection goes into a pinched up fatty layer on your abdomen or on the outer part of your thighs. Keep away from your belly button. Do NOT inject into a muscle.
Ideally you should inject at the same time every day; this helps to maintain a steady level of the medicine in your body.
When giving yourself an injection, make sure you:
1. Thoroughly wash and dry your hands.
2. Sit, stand or lie in a position so that you can see the skin where you are going to inject yourself. This can comfortably be done standing up, or if you prefer, in a lounge chair, recliner or bed propped up with pillows.
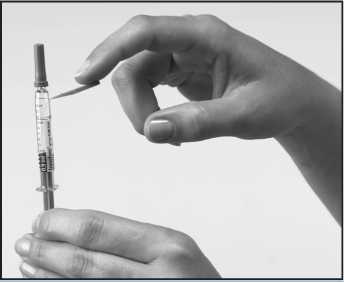
Carefully take the syringe out of its plastic container by bending the cap all the way back and sliding the syringe out. Bend the orange safety device down away from the cap on the needle. Remove the protective needle cap without bending the needle. To keep the needle clean, make sure it does not touch anything. The syringe is now ready for use.
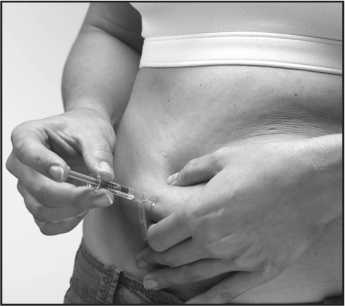
5. Hold the syringe in your writing-hand like you would hold a pen. With your other hand, make a fold of your skin by gently pinching the area where you are going to inject yourself with your thumb and forefinger.
6. With the syringe at a right angle to your body (pointing straight, not at an angle), insert the needle fully into the skin fold.
7. Continue to hold the skin fold, press down on the plunger slowly over 10-15 seconds. This delivers this medicine into the fatty tissue.
8. Pull the needle completely out of the skin and then let go of the skin fold. Do not rub or massage the place where you injected yourself-this can lead to bruising.
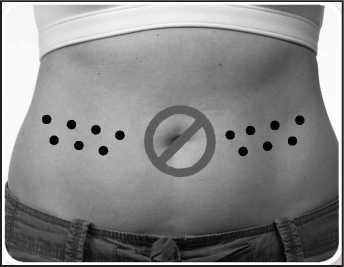
3. Decide where to inject yourself. This is usually on the right or left side of the abdomen remembering not to inject yourself within 5 cm (2 inches) of your belly button. You may also inject into the sides of your thigh. Do not inject near any scars or bruises. Each time you inject yourself, choose the opposite side from the site of your previous injection. So if you injected your right thigh last time, you would inject your left thigh next time. If you are injecting in your abdomen, you would do the left side one day and right the next. You should also avoid injecting into the exact site of a previous injection.
4. Clean the chosen area of the skin, as you have been told to do by your doctor or nurse, and allow to dry before you inject yourself.

J
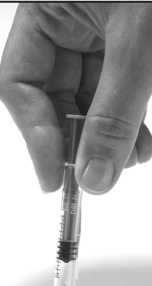
9. Using a hard surface, bend the orange safety device so it is now pointing in the same direction as the needle (back to its original position). Then with the safety device flat against a hard surface such as a table, gently push downwards until the needle clicks into the device. Then continue to push downwards against the hard surface, so that the needle and device are at a 45 degree angle to the syringe.
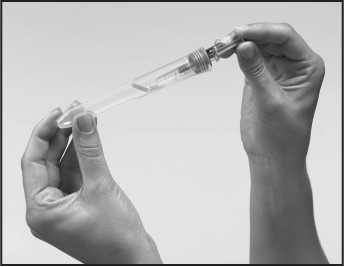
10. The used syringe, even when the orange safety device is in place, should be handled with care and should be disposed of in a "sharps" container (i.e. a special bin for needles) immediately. If a "sharps" container is not readily available then please put the used syringe back into the plastic container and close the lid by pressing down on the lid until it clicks into the slot provided. Dispose of the syringe carefully, as instructed by your healthcare professional. Never put syringes or needles in the household rubbish.
185 mm


For the attention of the healthcare professional:
Please dispose of the used syringe in accordance with your institution/employer's standard procedures for disposal of used syringes.
If you use more innohep than you should
If you think you may have injected yourself with too much, tell your doctor or nurse straight away because you may start to haemorrhage (bleed severely) and need to be given another injection of a medicine called protamine sulphate to stop you bleeding.
If you have missed a dose of innohep
If you forget to have your injection, it is important that you talk to your doctor or nurse as soon as you remember and get advice on what to do.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The side effects most often reported are blood problems and skin reactions, especially where your injection has been given.
Important side effects to look out for:
You must get urgent medical help if you have any of the following symptoms. You may be having a serious allergic reaction. These are rare (may affect 1 in 1000 people).
• You have difficulty breathing.
• Your face or throat swell.
• Your skin develops a severe rash.
• You experience blistering of the skin, mouth, eyes or genitals or your skin peels.
• Blood spots near the injection site which could develop into a purple blister surrounded by red inflamed skin.
You must get urgent medical help if you have any of the following symptoms after having an epidural or spinal anaesthetic. You may be developing paralysis:
• Tingling, weakness or numbness in your legs or lower body.
• Back pain.
• Problems in going to the toilet.
You should tell your doctor straight away if you spot any of the following signs which mean you may be starting to bleed severely:
• Red or brown urine.
• Black tarry stools.
• Unusual bruising.
• Bleeding from your nose, or mouth or any operation wound that will not stop.
Common side effects (may affect up to 1 in 10 people)
• Bleeding (haemorrhage).
• Anaemia. Reduction in red blood cells which can make the skin pale and cause weakness and breathlessness.
• A pooling of blood in tissues which may result in the skin appearing dark in colour, similar to a large bruise.
• Pain, itching, bruising or bleeding, redness, swelling, nodules or hard lumps under your skin where the injection was given.
Uncommon side effects (may affect up to 1 in 100 people)
• Changes in your blood test results. There may be a change in the clotting cells (platelets) in your blood. These tests will return to normal when innohep is stopped.
• An allergic reaction. You may be sensitive to one of the ingredients in this medicine.
• Bruising, red or purple spots under your skin.
• Some blood tests may also show a change in the way your liver is working. These tests will return to normal when innohep is stopped.
• An itchy red rash with heat and swelling on your skin (dermatitis).
• Rash.
• Itchy skin.
Rare side effects (may affect up to 1 in 1000 people)
• Your blood may form more harmful clots. A drop in the number of clotting cells (platelets) in your blood may give you these symptoms. Your doctor can explain this more.
• Changes in your blood test results. The amount of potassium may be increased. This is more likely to happen if you have severe kidney problems or diabetes. Your doctor can explain this more.
• Hives.
• Your bones may weaken and break more easily. This is known as osteoporosis and has been seen in patients using heparin for a long time.
• Prolonged, painful erections in men.
Paediatric population
Limited information derived from one study and postmarketing data indicates that the pattern of adverse reactions in children and adolescents is comparable to that in adults.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store innohep®
• Keep this medicine out of the sight and reach of children.
• Do not use the medicine after the expiry date which is stated on the label (EXP). The expiry date is the last day of that month.
• Do not store above 25°C.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of pack and other information What innohep contains
• The active substance is tinzaparin sodium.
• innohep syringe 10,000 lU/ml contains 10,000 IU of tinzaparin sodium in each millilitre (ml).
• The other ingredients are sodium acetate, sodium hydroxide and water for injections.
You can find important information about some of the ingredients near the end of section 2, just before section 3.
What innohep looks like and contents of the pack
innohep is a straw coloured liquid.
innohep comes in glass syringe containing 0.25 ml, 0.35 ml or 0.45 ml.
There are 5,10, 50 or 100 syringes in a carton.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder: LEO Laboratories Limited, Hurley, Berkshire SL6 6RJ, UK.
Manufacturer: Laboratoires LEO, 28500 Vernouillet, France. This leaflet was last revised in February 2015.
® Registered Trade Mark
LEO
031279-XX
185 mm