Tobradex Eye Drops Suspension
Tobradex® Eye Drops, Suspension
(Tobramycin and Dexamethasone)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or your pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them even if their symptoms are the same as yours.
• If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
This product is available using the above name but will be referred to as Tobradex throughout this leaflet.
Patient Information Leaflet
In this leaflet:
1) What Tobradex is and what it is used for
2) What you need to know before you use Tobradex
3) How to use Tobradex
4) Possible side effects
5) How to store Tobradex
6) Content of the pack and other information
1) What Tobradex is and what it is used for
Tobradex contains dexamethasone, a corticosteroid and tobramycin, an antibiotic which is active against a wide range of bacteria that may infect the eye.
It is used to prevent and treat inflammation and prevent possible infection of the eye after cataract surgery in adults and children aged 2 years and older.
2) What you need to know before you use Tobradex Do not use Tobradex...
• If you have or think that you have any type of infection of the eye. Use of corticosteroids may make infections worse.
• If you have a sticky discharge from your eye.
• If you have a red eye that has not been seen by a doctor.
• If you are allergic to tobramycin or dexamethasone or to any of the other ingredients listed in section 6.
If any of these apply ask your doctor for advice.
Warnings and precautions
• If you have a disorder causing a thinning of the eye tissues, such as rheumatoid arthritis, Fuch's dystrophy or following a corneal transplant. Corticosteroids may cause further thinning and possible perforation.
• If you experience allergic reactions such as eyelid itching, swelling or, redness of the eye with Tobradex, discontinue use and consult your doctor. This allergic sensitivity may occur with other topical or systemic antibiotic of aminoglycoside type.
• If your symptoms get worse or suddenly return, please consult your doctor. You may become more susceptible to eye infections with the use of this product.
• If you are using other antibiotic treatment, including oral, with Tobradex, ask your doctor for advice.
• If you use Tobradex for a long period of time, you may become more susceptible to eye infections, have increased pressure in your eye( s ) or develop cataracts.
You may still be able to use Tobradex, but discuss it with your doctor first.
• Intraocular pressure should be checked frequently, this is especially important in children below 6 years of age receiving dexamethasone-containing products.
• Do not give Tobradex to children below 2 years old because the safety and efficacy in this population has not been established.
Other medicines and Tobradex
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
If you are using other eye drops or eye ointments, wait at least 5 minutes between using each one. Eye ointments should be administered last.
Pregnancy and breast-feeding
If you are pregnant or might get pregnant, or if you are breast-feeding a baby, talk to your doctor before you use Tobradex.
Driving and using machines
If you experience temporary blurred vision after using Tobradex you should not drive or operate machinery until your vision is clear.
Important information if you wear Contact Lenses
Contact lens wear is not recommended during treatment of an ocular infection or inflammation.
Benzalkonium chloride, used as a preservative in Tobradex, may cause eye irritation and discolour soft contact lenses.
If you wear soft contact lenses remove them before using Tobradex and wait at least 15 minutes before putting them back in.
3) How to use Tobradex
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The usual dose
The usual dose is 1 drop in the affected eye(s) every 4 to 6 hours while you are awake.
During the initial 48 hours, your doctor may increase the dose to 1 drop every 2 hours.
Do not use for more than 24 days.
Tobradex may be used in children 2 years of age and older at the same dose as in adults.
Remove the loose collar from the cap when the bottle is first opened. Always use Tobradex exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
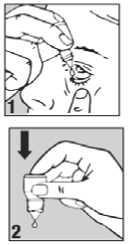
How to use
• Wash your hands before you start.
• Shake the bottle well.
• Twist off the bottle cap.
• Hold the bottle pointing down, between your thumb and fingers.
• Tilt your head back.
• Pull down your lower eyelid with a finger, until there is a 'pocket' between the eyelid and your eye. The drop will go in here (picture 1).
• Bring the bottle tip close to the eye. Do this in front of a mirror if it helps.
• Do not touch your eye or eyelid, surrounding areas or other surfaces with the dropper. It could infect the drops.
• Gently press on the base of the bottle to release one drop at a time (picture 2).
• Do not squeeze the bottle, only a gentle press on the bottom is needed.
• If you use drops in both eyes, repeat the steps for your other eye. Put the bottle cap firmly back on immediately after use.
• If a drop misses your eye, try again.
• If you forget to take Tobradex, do not worry, just take it as soon as possible. Do not take a double dose to make up.
• If you use more Tobradex than you should it can be washed out with warm water.
If you have any further questions on the use of Tobradex, ask your doctor or pharmacist.
4) Possible side effects
Like all medicines, Tobradex can cause side effects, although not everybody gets them.
The following side effects have been seen with Tobradex
Uncommon side effects
(may affect up to 1 in 100 people )
Effects in the eye: increased pressure in your eye(s), eyelid redness, eyelid swelling, eye irritation, eye pain, eye itching, watery eyes, eye discomfort.
General side effects: headache, runny nose, tightness of the throat.
Rare side effects
(may affect up to 1 in 1000 people)
Effects in the eye: redness, blurred vision, dry eye, eye allergy, eye surface inflammation.
General side effects: bad taste.
Not known (frequency cannot be estimated from available data )
Effects in the eye: increase in pupil size, eyelid redness, increased tear production, eye surface inflammation.
General side effects: allergy (hypersensitivity), dizziness, nausea, abdominal discomfort, rash, swelling of the face, itching.
If Tobradex is used for more than 24 days, it may cause you to get an
infection and the healing of your wound may also be delayed.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system (see details below).
By reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
5) How to store Tobradex
• Keep out of the sight and reach of children.
• Do not store above 25°C. Do not refrigerate or freeze.
• Do not use this medicine after the expiry date (shown as "EXP" on the bottle and carton).
• Once the bottle has been opened then this medicine must be thrown away four weeks after opening.
• Any leftover or out of date drops should be returned to your pharmacist who will dispose of them safely for you.
• If the eye drops become discoloured or show signs of any deterioration, you should seek the advice of your pharmacist.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
• Do not pass this medicine on to others. It may harm them, even if their symptoms are the same as yours.
6) Further information What Tobradex contains
Each ml of Tobradex Eye Drops, Suspension contains 1mg of dexamethasone and 3mg of tobramycin as the active ingredients.
They also contain benzalkonium chloride, disodium edetate, tyloxapol, sodium chloride, sodium sulphate, hydroxyethylcellulose and purified water. Tiny amounts of sulphuric acid or sodium hydroxide are sometimes added to keep acidity levels (pH levels) normal.
What Tobradex looks like and contents of the pack
Tobradex Eye Drops is an eye drop suspension packaged in a 5-ml white low density plastic bottle, with a dispensing nozzle and a white tamper evident polypropylene closure.
PL 10383/1805 Tobradex® Eye Drops, Suspension |POM|
Who makes and repackages your medicine?
Your medicine is manufactured by Alcon Cusi S.A. Camil Fabra 58, El Masnou, Barcelona Spain. Procured from within the EU and repackaged by Product Licence Holder: Primecrown Ltd, 4/5 Northolt Trading Estate, Belvue Road, Northolt, Middlesex, UB5 5QS.
Leaflet date: 31.03.2015
Tobradex is a registered trademark of Novartis AG, Basle, Switzerland.