Tobradex Eye Drops
Out of date information, search another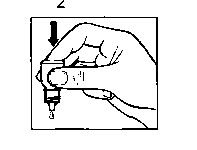

S0071-5-RM-PIL-19.02.2013
PATIENT INFORMATION LEAFLET
TOBRADEX® EYE DROPS, SUSPENSION (tobramycin/dexamethasone)
The name of your medicine is Tobradex Eye Drops, Suspension, but will be referred to as Tobradex throughout the remainder of this leaflet.
Read all of this leaflet carefully before you start using this medicine
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or your pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them even if their symptoms are the same as yours.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet
1. What Tobradex is and what it is used for
2. Before you use Tobradex
3. How to use Tobradex
4. Possible side effects
5. How to store Tobradex
6. Further information
1. What Tobradex is and what it is used for
Tobradex contains dexamethasone, a corticosteroid and tobramycin, an antibiotic which is active against a wide range of bacteria that may infect the eye.
It is used to prevent and treat inflammation and possible infection of the eye after cataract surgery in adults and children 2 years and older.
2. Before you use Tobradex Do not use Tobradex
• If you have or think that you have any type of infection of the eye.
Use of corticosteroids may make infections worse.
• If you have a sticky discharge from your eye.
• If you have a red eye that has not been seen by a doctor.
• If you are allergic to tobramycin or dexamethasone or to any of the other ingredients listed in section 6.
If any of these apply ask your doctor for advice.
Take special care
• If you have a disorder causing a thinning of the eye tissues, such as rheumatoid arthritis, Fuch’s dystrophy or following a corneal transplant. Corticosteroids may cause further thinning and possible perforation.
You may still be able to use Tobradex, but discuss it with your doctor first.
• Intraocular pressure should be checked frequently, especially in children below 6 years of age receiving dexamethasone-containing products.
• Do not give Tobradex to children below 2 years old because the safety and efficacy in this population has not been established.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
If you are also using other eye drops, wait at least 10 minutes between using each one
Pregnancy and breast-feeding
If you are pregnant or might get pregnant, or if you are breast-feeding a baby, talk to your doctor before you use Tobradex.
Driving and using machines
If you experience temporary blurred vision after using Tobradex you should not drive or operate machinery until your vision is clear.
Important information if you wear Contact Lenses
Benzalkonium chloride, used as a preservative in Tobradex, may cause eye irritation and discolour soft contact lenses.
If you wear soft contact lenses remove them before using Tobradex and wait at least 15 minutes before putting them back in.
3. How to use Tobradex The usual dose
The usual dose is 1 drop in the affected eye(s) every 4 to 6 hours while you are awake. During the initial 48 hours, your doctor may increase the dose to 1 drop every 2 hours.
Do not use for more than 24 days.
Tobradex may be used in children 2 years of age and older at the same dose as in adults.
Remove the loose collar from the cap when the bottle is first opened.
Always use Tobradex exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
How to use
1
• Wash your hands before you start.
• Shake the bottle well.
• Twist off the bottle cap.
• Hold the bottle pointing down, between your thumb and fingers.
• Tilt your head back.
• Pull down your lower eyelid with a finger, until there is a 'pocket' between the eyelid and your eye. The drop will go in here (picture 1).
• Bring the bottle tip close to the eye. Do this in front of a mirror if it helps.
• Do not touch your eye or eyelid, surrounding areas or other surfaces with the dropper. It could infect the drops.
• Gently press on the base of the bottle to release one drop at a time (picture 2).
• Do not squeeze the bottle, only a gentle press on the bottom is needed.
• If you use drops in both eyes, repeat the steps for your other eye. Put the bottle cap firmly back on immediately after use.
• If a drop misses your eye, try again.
• If you forget to take Tobradex, do not worry, just take it as soon as possible. Do not take a double dose to make up.
• If you use more Tobradex than you should it can be washed out with warm water.
If you have any further questions on the use of Tobradex, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, Tobradex can cause side effects, although not everybody gets them.
• You may experience some or all of the following effects in your eye(s):
Uncommon (affect 1 to 10 people in 1000): Irritation, pain, itching, redness, watery eyes, discomfort, feeling of something in your eye, blurred vision, dry eye, raised pressure in your eye, which may lead to headaches and disturbances of vision.
Swelling and redness of the eyelid and sensitivity to light may also occur.
• You may also experience effects in other areas of your body including:
Uncommon: Headache, runny nose, tightness of the throat.
If Tobradex is used for more than 24 days, it may make you more likely to get an infection and the healing of your wound may also be delayed.
If any of the side effects get serious, or you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
TURN OVER
5. How to store Tobradex
• Keep out of the sight and reach of children.
• Do not store above 25°C.
• Do not refrigerate or freeze.
• Store in the original package.
• Keep the bottle tightly closed.
• Do not use the drops after the expiry date which is stated on the bottle and the carton after ‘Exp:' The expiry date refers to the last day of that month.
• Stop using the bottle 4 weeks after first opening, to prevent infections.
• Medicines should not be disposed of via waste water or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
• Do not pass this medicine on to others. It may harm them, even if their symptoms are the same as yours
• If you notice any signs of discolouration or deterioration of this medicine return it to your pharmacist.
6. Further Information What Tobradex contains?
Each ml of suspension contains 1mg of dexamethasone and 3mg of tobramycin as the active ingredients.
Tobradex also contains benzalkonium chloride, disodium edetate, tyloxapol, sodium chloride, sodium sulphate anhydrous, hydroxyethyl cellulose, sulphuric acid or sodium hydroxide (to adjust pH) and purified water.
What Tobradex looks like and contents of the pack
Tobradex is a white plastic dropper bottle, with a white plastic screw-cap and tamper evident ring. The bottle contains a white to off-white suspension. Pack size: 5ml.
Manufacturer: SA Alcon-Couvreur NV, Rijksweg 14, B-2870 Puurs, Belgium.
Procured from within the EU and repackaged by: Amimed Direct Ltd, Hendon, London, NW9 6AQ.
Product License Holder: Sam Pharma Ltd, Unit 20 Garrick Industrial Estate, Irving Way, Hendon, London, NW9 6AQ.
PL No: 33902/0071
Leaflet revised: 19.02.2013
Tobradex® is a registered trademark of Alcon Inc.
S0071-5-RM-PIL-19.02.2013