Transtec 35 Micrograms-H Transdermal Patch
PACKAGE LEAFLET: INFORMATION FOR THE USER
Transtec® 35 micrograms/h / 52.5 micrograms/h / 70 micrograms/h transdermal patch
(buprenorphine)
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
This medicine is known as Transtec 35 micrograms/h / 52.5 micrograms/h / 70 micrograms/h transdermal patch, but will be referred to as Transtec.
What is in this leaflet
1. What Transtec is and what it is used for
2. What you need to know before you use Transtec
3. How to use Transtec
4. Possible side effects
5. How to store Transtec
6. Contents of the pack and other information
1. What TRANSTEC is and what it is used for
Transtec is an analgesic (a pain-relieving medicine) intended to relieve moderate to severe cancer pain and severe pain that has not responded to other types of painkillers. Transtec acts through the skin. When the transdermal patch is applied to the skin, the active substance buprenorphine passes through the skin into the blood. Buprenorphine is an opioid (strong pain reliever), which reduces pain by acting on the central nervous system (specific nerve cells in the spinal cord and in the brain). The effect of the transdermal patch lasts for up to four days. Transtec is not suitable for the treatment of acute (short-lasting) pain.
2. What you need to know before you use TRANSTEC Do not use Transtec:
- if you are allergic to buprenorphine or any of the other ingredients of this medicine (listed in section 6);
- if you are dependent on strong pain relievers (opioids);
- if you suffer from a disease in which you have or may have great difficulty breathing
- if you are taking monoamine oxidase (MAO) inhibitors (certain medicines used to treat depression) or you have taken this type of medicine in the last two weeks (see “Other medicines and Transtec”);
- if you suffer from myasthenia gravis (a certain type of severe muscle weakness);
- if you suffer from delirium tremens (confusion and trembling caused by abstinence from alcohol following habitual excessive drinking or occurring during an episode of heavy alcohol consumption);
- if you are pregnant.
Transtec must not be used to treat withdrawal symptoms in drug-dependent persons.
Warnings and precautions
Talk to your doctor or pharmacist before using Transtec
- if you have recently drunk a lot of alcohol;
- if you suffer from seizures or convulsions (fits)
- if your consciousness is disturbed (feeling light-headed or faint) for an unknown reason;
- if you are in a state of shock (cold sweat might be a sign of it);
- if the pressure in your skull is increased (for instance after head injury or in brain disease), and artificial respiration is not possible;
- if you have difficulty breathing or are taking other medicines that may make you breathe more slowly or weakly (see “Other medicines and Transtec”);
- if your liver does not work properly;
- if you are inclined to abuse medicines or drugs.
Also, please be aware of the following precautions:
- Some people may become dependent on strong pain relievers such as Transtec when they use them over a long period of time. They may have withdrawal effects when they stop using them (see “If you stop using Transtec”).
- Fever and external heat may lead to larger quantities of buprenorphine in the blood than normal. Also, external heat may prevent the transdermal patch from sticking properly. Therefore, do not expose yourself to external heat (e.g. sauna, infra-red lamps, electric blankets, hot water bottles) and consult your doctor if you have fever.
Athletes should be aware that this medicine may cause a positive reaction to sports doping control tests.
Children and adolescents
Transtec should not be used in persons below the age of 18 years, because no experience has so far been gained in this age group. Other medicines and Transtec
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
- Transtec must not be used together with monoamine oxidase (MAO) inhibitors (certain medicines used to treat depression), or if you have taken this type of medicine for the last 2 weeks.
- Transtec may make some people feel drowsy, sick, or faint or make them breathe more slowly or weakly. These side effects may be intensified if other medicines that may produce the same effects are taken at the same time. These other medicines include other strong pain relievers (opioids), certain sleeping pills, anaesthetics, and medicines used to treat certain psychological diseases such as tranquillizers, anti-depressants, and neuroleptics.
- If Transtec is used together with some medicines, the effects of the transdermal patch may be increased. These medicines include e.g. certain anti-infectives/anti-fungals (e.g. containing erythromycin or ketoconazole) or HIV medicines (e.g. containing
ritonavir)
- If Transtec is used together with other medicines, the effects of the transdermal patch may be reduced. These medicines include certain products, e.g. dexamethasone; medicines to treat epilepsy (e.g. containing carbamazepine, or phenytoin) or medicines for tuberculosis (e.g. rifampicin).
Transtec with food, drink and alcohol
You should not drink alcohol while using Transtec. Alcohol may intensify certain side effects of the transdermal patch and you may feel unwell. Drinking grapefruit juice may intensify the effects of Transtec.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
There is not sufficient experience regarding the use of Transtec in pregnant women. Therefore do not use Transtec if you are pregnant.
Buprenorphine, the active substance contained in the transdermal patch, inhibits milk formation and passes into the breast milk. Therefore, do not use Transtec if you are breast-feeding.
Driving and using machines
Transtec may make you feel dizzy or drowsy or experience blurred or double vision and affect your reactions to such an extent that you may not react adequately or quickly enough in the event of unexpected or sudden occurrences. This applies particularly
- at the beginning of treatment,
- when your dosage is changed,
- when you switch to Transtec from another pain reliever,
- if you also use other medicines that act on the brain,
- if you drink alcohol.
If you are affected, you should not drive or operate machinery whilst using Transtec. This applies also at the end of treatment with Transtec. Do not drive or operate machinery for at least 24 hours after the patch has been removed.
Discuss with your doctor or pharmacist if you are unsure about anything.
3. How to use TRANSTEC
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Transtec is available in three strengths: Transtec 35 micrograms/h transdermal patch, Transtec 52.5 micrograms/h transdermal patch and Transtec 70 micrograms/h transdermal patch.
The choice of which strength of Transtec will suit you best will be made by your doctor. During treatment your doctor may change which transdermal patch you use to a smaller or larger one if necessary.
The recommended dose is:
Adults
Unless your doctor has told you differently, attach one Transtec transdermal patch (as described in detail below) and change it after 4 days at the latest. For convenience of use, you can change the transdermal patch twice a week at the same days, e.g. always on Monday mornings and Thursday evenings. To help you remember when to change your transdermal patch, you should make a note on the calendar on the outer packaging. If your doctor has advised you to take other pain relievers in addition to the transdermal patch, strictly follow the doctor‘s instructions, otherwise you will not fully benefit from treatment with Transtec.
Use in children and adolescents
Transtec should not be used in persons below the age of 18 years, because no experience has so far been gained in this age group. Elderly patients
No dosage adjustment is needed for elderly patients.
Patients with kidney disease / dialysis patients
In patients with kidney disease and in dialysis patients, no dosage adjustment is necessary.
Patients with liver disease
of Transtec may be affected. If this applies to you, your doctor will
In patients with liver disease, the intensity and duration of action check on you more closely.
Method of administration
Before applying the transdermal patch
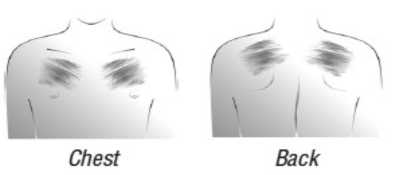
- Choose an area of skin which is flat, clean and hairless on your upper body, preferably on the chest below the collar-bone or on the upper part of the back (see adjacent illustrations). Call assistance if you cannot apply the transdermal patch yourself.
- If the chosen area has hairs, cut them off with a pair of scissors. Do not shave them off!
- Avoid skin which is red, irritated or has any other blemishes, for instance large scars.
- The area of skin you choose must be dry and clean. If necessary, wash it with cold or lukewarm water. Do not use soap or other detergents. After a hot bath or shower, wait until your skin is completely dry and cool. Do not apply lotion, cream or ointment to the chosen area. This might prevent your transdermal patch from sticking properly.
Applying the transdermal patch:
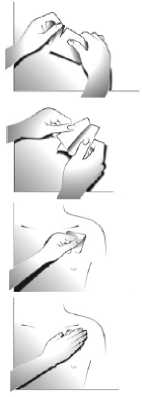
Step 1:
Each transdermal patch is sealed in a sachet. Just before use, open the sachet by tearing at the notch. Take out the transdermal patch.
Step 2:
The sticky side of the transdermal patch is covered with a silvery protective foil. Carefully peel off half the foil. Try not to touch the sticky part of the transdermal patch.
Step 3:
Stick the transdermal patch onto the area of skin you have chosen and remove the remaining foil.
Step 4:
Press the transdermal patch against your skin with the palm of your hand for about 30 seconds. Make sure that the whole transdermal patch is in contact with your skin, especially at the edges.
Wearing the transdermal patch
You may wear the transdermal patch for up to 4 days. Provided that you have applied the transdermal patch correctly, there is little risk of it coming off. You may shower, bathe or swim while wearing it. However, do not expose the transdermal patch to extreme heat (e.g. sauna baths, infra-red lamps, electric blankets, hot water bottles).
In the unlikely event that your transdermal patch falls off before it needs changing, do not use the same transdermal patch again. Stick a new one on straight away (see “Changing the transdermal patch" below).
Changing the transdermal patch
- Take the old transdermal patch off.
- Fold it in half with the sticky side inwards.
- Throw it away carefully, out of the sight and reach of children.
- Stick a new transdermal patch on a different skin site (as described above). Wait at least one week before using the same site again. Duration of treatment
Your doctor will tell you how long you may use Transtec. Do not stop using Transtec on your own account, because pain may return and you may feel unwell (see also “If you stop using Transtec“ below).
If you have the impression that the effect of the Transtec transdermal patch is too weak or too strong, tell your doctor or pharmacist.
If you use more Transtec than you should
If this happens there may be signs of an overdose of the substance buprenorphine. An overdose may intensify the side effects of buprenorphine such as drowsiness, nausea, and vomiting. You may get pin-point pupils and breathing may become slow and weak. You may also get cardiovascular collapse.
As soon as you discover that you have used more transdermal patches than you should, remove the excess transdermal patches and talk to a doctor or pharmacist.
If you forget to use Transtec
If you forget an application, stick a new transdermal patch on as soon as you remember. You will then need to change your routine, e.g. if you usually apply your transdermal patches on Mondays and Thursdays, but you forget and don't stick on a new transdermal patch until Wednesday, you will need to change your transdermal patches on Wednesdays and Saturdays from then on. Make a note of the new pair of days on the calendar on the outer packaging. If you are very late changing your transdermal patch, pain may return. In this case please contact your doctor.
Never apply twice the number of transdermal patches to make up for the forgotten application!
If you stop using Transtec
If you interrupt or finish using Transtec too soon, pain may return. If you wish to stop use on account of unpleasant side effects, please consult your doctor. He/she will tell you what can be done and whether you can be treated with other medicines.
Some people may experience withdrawal-effects when they have used strong pain relievers for a long time and stop using them. The risk of having effects after you stop using Transtec is very low. However, if you feel agitated, anxious, nervous or shaky, if you are overactive, have difficulty sleeping or digestion problems, tell your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects are classified as follows:
|
Very common: more than 1 out of 10 persons |
Common: more than 1 out of 100 persons and less than 1 out of 10 persons |
|
Uncommon: |
Rare: |
|
more than 1 out of 1,000 persons |
more than 1 out of 10,000 persons |
|
and less than 1 out of 100 persons |
and less than 1 out of 1,000 persons |
|
Very rare: | |
|
less than 1 out of 10,000 persons |
The following side effects have been reported:
Immune system disorders
Very rare: serious allergic reactions (see below)
Metabolism and nutrition disorders
Rare: loss of appetite
Psychiatric disorders
Uncommon: confusion, sleep disorder, restlessness
Rare: illusions such as hallucinations, anxiety and nightmares, reduced sex drive
Very rare: dependence, mood swings
Nervous system disorders
Common: dizziness, headache
Uncommon: various degrees of sedation (calmness), ranging from tiredness to muzziness
Rare: difficulty concentrating, speech disorder, muzziness, disturbed balance, abnormal skin sensations
(numbness, prickling or burning sensations)
Very rare: muscle twitching, taste disorders
Eye disorders
Rare: visual disturbance, blurred vision, swollen eyelids
Very rare: pin-point pupils
Ear disorders
Very rare: ear pain
Heart and blood circulation disorders
Uncommon: circulatory disorders (such as low blood pressure or, rarely, even
circulatory collapse)
Rare: hot flushes
Chest and lung disorders
Common: shortness of breath
Rare: difficulty breathing (respiratory depression)
Very rare: abnormally rapid breathing, hiccups
Digestive system disorders
Very common: Common: Uncommon: Rare:
Very rare:
nausea (feeling sick) vomiting, constipation dry mouth heartburn retching
Skin disorders
Very common: Common: Uncommon: Rare:
Very rare:
(generally at the site of application)
redness, itching
skin changes (exanthema, generally on repeated use), sweating
rash
hives
pustules, small blisters
Urinary system disorders
Uncommon: difficulty in passing water, urine retention (less urine than normal)
Reproductive system disorders
Rare: erection difficulties
General disorders
Common: oedema (e.g. swelling of the legs), tiredness
Uncommon: weariness
Rare: withdrawal symptoms (see below), administration site reactions
Very rare: chest pain
If you notice any of the side effects listed above, tell your doctor as soon as possible.
In some cases delayed allergic reactions occurred with marked signs of inflammation. in such a case you should stop using Transtec after you have talked to your doctor.
If you experience swelling of the hands, feet, ankles, face, lips, mouth, or throat which may cause difficulty in swallowing or breathing, hives, fainting, yellowing of the skin and eyes (also called jaundice), remove the transdermal patch and call your doctor immediately or seek help at the casualty department of the nearest hospital. These can be symptoms of a very rare serious allergic reaction.
Some people may have withdrawal symptoms when they have used strong pain relievers for a long time and stop using them. The risk of having withdrawal effects when you stop using Transtec is low. However, if you feel agitated, anxious, nervous or shaky, if you are overactive, have difficulty sleeping or digestion problems, tell your doctor.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via: Yellow Card Scheme, at: www.mhra.gov.uk/yellowcard
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store TRANSTEC
Keep out of the sight and reach of children.
Do not use Transtec after the expiry date which is stated on the carton and on the sachet after “Expiry date (month/year):“. The expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
If your patches become discoloured or show any other signs of deterioration, you should seek the advice of your pharmacist who will tell you what to do.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information What Transtec contains
The active substance in Transtec is buprenorphine.
|
Transtec 35 micrograms/h transdermal patch |
Each transdermal patch contains 20 mg buprenorphine and releases about 35 micrograms of buprenorphine per hour. The area of the transdermal patch containing the active substance is 25 cm2. |
|
Transtec 52.5 micrograms/h transdermal patch |
Each transdermal patch contains 30 mg buprenorphine and releases about 52.5 micrograms of buprenorphine per hour. The area of the transdermal patch containing the active substance is 37.5 cm2. |
|
Transtec 70 micrograms/h transdermal patch |
Each transdermal patch contains 40 mg buprenorphine and releases about 70 micrograms of buprenorphine per hour. The area of the transdermal patch containing the active substance is 50 cm2. |
The other ingredients in Transtec are:
Polyethylene terephthalate siliconised and aluminised foil, Polyethylene terephthalate web, Povidone K90, 4-Oxopentanoic acid, Oleyl oleate, Polyethylene terephthalate film, Duro-tak 387- 2051 with crosslinker aluminium actylacetonate, Duro-tak 387-2054 including crosslinker.
What Transtec looks like and contents of the pack
Transtec transdermal patches are skin-coloured with rounded corners and are imprinted
Transtec 35 |jg/h buprenorphinum 20 mg in dark purple ink Transtec 52.5 jg/h buprenorphinum 30 mg in green ink Transtec 70 jg/h buprenorphinum 40 mg in red ink
Transtec comes in cartons containing 3 or 4 transdermal patches individually sealed in sachets.
Manufacturer
LTS Lohmann Therapie-Systeme GmbH-Germany.
Procured from within the EU. Product Licence Holder and repackaged by: S.C.A.C. Ltd., Unit 2A, Bandeath Industrial Estate, Throsk, Stirling, FK7 7NP, United Kingdom.

Transtec 35 micrograms/h Transdermal patch PL: 30984/0195 Transtec 52.5 micrograms/h Transdermal patch PL: 30984/0196 Transtec 70 micrograms/h Transdermal patch PL: 30984/0197
This leaflet was last approved in 18 August 2015
Transtec® is a registered trademark of Grunenthal GmbH.
REV_3