Travoprost 40 Micrograms/Ml Eye Drops Solution

PACKAGE LEAFLET: INFORMATION FOR THE USER
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions ask your doctor or pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet, see section 4.
WHAT IS IN THIS LEAFLET:
1. What Travoprost is and what it is used for
2. What you need to know before you use Travoprost
3. How to use Travoprost
4. Possible side effects
5. How to store Travoprost
6. Contents of the pack and other information
Ol WHAT TRAVOPROST IS AND WHAT IT IS USED FOR
Travoprost contains travoprost, one of a group of medicines called prostaglandin analogues. It works by reducing the pressure in the eye. It may be used on its own or with other drops e.g. beta-blockers, which also reduce pressure.
Travoprost eye drops are used to treat high pressure in the eye in adults, adolescents and children from 2 months onward. This pressure can lead to an illness called glaucoma.
©I WHAT YOU NEED TO KNOW BEFORE YOU USETRAVOPROST
Do not use Travoprost
• If you are allergic to travoprost or any of the other ingredients of this medicine (listed in section 6).
Ask your doctor for advice if this applies to you.
Warnings and precautions
• Travoprost may increase the length, thickness, colour and/or number of your eyelashes. Changes in the eyelids including unusual hair growth or in the tissues around the eye have also been observed
• Travoprost may change the colour of your iris (the coloured part of your eye). This change may be permanent. A change in the colour of the skin around the eye may also occur • If you have had cataract surgery, talk to your doctor before you use travoprost • If you have current or previous history of an eye inflammation (iritis and uveitis), talk to your doctor before you use travoprost • Travoprost may rarely cause breathlessness or wheezing or increase the symptoms of asthma. If you are concerned about changes in your breathing pattern when using travoprost advise your doctor as soon as possible • Travoprost may be absorbed through the skin. If any of the medicinal product comes into contact with the skin, it should be washed off straight away. This is especially important in women who are pregnant or are attempting to become pregnant
• If you wear soft contact lenses, do not use the drops with your lenses in. After using the drops wait 15 minutes before putting your lenses back in.
Children and adolescents
Travoprost can be used in children from 2 months to < 18 years at the same dose as for adults. Use of Travoprost is not recommended to those children under 2 months of age.
Other medicines and Travoprost
Tell your doctor or pharmacist if you are taking or have recently taken or used any other medicines.
Pregnancy, breast-feeding and fertility
Do not use travoprost if you are pregnant. If you think that you may be pregnant speak with your doctor right away. If you could become pregnant you must use adequate contraception whilst you use travoprost.
Do not use travoprost if you are breast-feeding, travoprost may get into your milk.
Ask your doctor for advice before taking any medicine.
Driving and using machines
You may find that your vision is blurred for a time just after you use travoprost. Do not drive or use machines until this has worn off.
Travoprost contains benzalkonium chloride If you wear soft contact lenses. Do not use the drops with your lenses in. Wait 15 minutes after using the drops before putting your lenses back in. There is a preservative in Travoprost (benzalkonium chloride) that can discolour soft lenses.
This product contains benzalkonium chloride.
Patients who can not tolerate benzalkonium chloride may use other travoprost eye drops, containing different preservative agent. Benzalkonium chloride may cause small breaks in the surface of the eye.
Travoprost contains macrogol glycerol hydroxy stearate
This may cause skin reactions.
^ HOW TO USE TRAVOPROST
Always use this medicine exactly as your doctor or the doctor treating your child has told you. You should check with your doctor, the doctor treating your child or pharmacist if you are not sure.
The recommended dose is
One drop in the affected eye or eyes, once a day-in the evening.
Only use travoprost in both eyes if your doctor told you to. Use it for as long as your doctor or the doctor treating your child told you to.
Only use travoprost for dropping in your or your child’s eye(s).
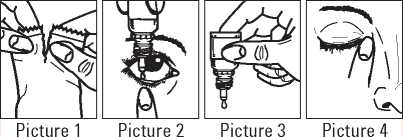
• Immediately before using a bottle for the first time, tear open the overwrap pouch, take the bottle out (Picture 1) and write the date of opening on the label in the space provided
• Wash your hands
• Twist off the cap
• Hold the bottle, pointing down, between your thumb and fingers
• Tilt your head or your child’s head gently back. Pull down the eyelid with a clean finger, until there is a 'pocket’ between the eyelid and the eye. The drop will go in here (Picture 2)
• Bring the bottle tip close to the eye. Use a mirror if it helps
• Do not touch the eye or eyelid, surrounding areas or other surfaces with the dropper. It could infect the drops
• Gently squeeze the bottle to release one drop of travoprost at a time (Picture 3)
• After using travoprost, keep the eyelid closed, apply gentle pressure by pressing a finger into the corner of the eye, by the nose (Picture 4) for at least 1 minute. This helps to stop travoprost getting into the rest of the body

• If you use drops in both eyes, repeat the steps for the other eye
• Close the bottle cap firmly immediately after use
• Only use one bottle at a time. Do not open the pouch until you need to use the bottle.
If a drop misses the eye, try again.
If you or your child are using other eye preparations
such as eye drop or eye ointment, wait for at least 5 minutes between putting in travoprost and the other eye preparations.
If you or your child receive more Travoprost than you should
Rinse all the medicine out with warm water. Don’t put in any more drops until it’s time for your next regular dose.
If you forget to use Travoprost
Continue with the next dose as planned. Do not use a double dose to make up for a forgotten dose. Never use more than one drop in the affected eye(s) in a single day.
If you stop using Travoprost
Do not stop using Travoprost without first speaking to your doctor or the doctor treating your child, the pressure in your eye or your child’s eye will not be controlled which could lead to loss of sight.
If you have any further questions on the use of this product, ask your doctor, the doctor treating your child or pharmacist.
^ POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects although not everybody gets them.
You can usually carry on using the drops, unless the side effects are serious. If you’re worried, talk to a doctor or pharmacist. Do not stop taking travoprost without speaking to your doctor.
The following side effects have been seen with travoprost
Very common: may affect more than 1 in 10 people Effects in the eye: eye redness.
Common: may affect up to 1 in 10 people Effects in the eye: changes in the colour of the iris (coloured part of the eye), eye pain, eye discomfort, dry eye, itchy eye, eye irritation.
Uncommon: may affect up to 1 in 100 people Effects in the eye: corneal disorder, eye inflammation, iris inflammation, inflammation inside the eye, eye surface inflammation with/out surface damage, sensitivity to light, eye discharge, eyelid inflammation, eyelid redness, swelling around the eye, eyelid itching, reduced vision, blurred vision, increased tear production, infection or inflammation of the conjunctiva (conjunctivitis), abnormal turning outward of the lower eyelid, clouding of the eye, eyelid crusting, growth of eyelashes, discolouration of the eyelashes, tired eyes.
General side effects: increased allergic symptoms, headache, dizziness, feeling your heart beat, shortness of breath, asthma, stuffy nose, throat irritation, darkening of skin around the eye(s), skin darkening, abnormal hair texture, excessive hair growth, visual field defect.
Rare: may affect up to 1 in 1000 people Effects in the eye: perception of flashing lights, eczema of the eyelids, eye swelling, halo vision, decreased eye sensation, inflammation of the glands of the eyelids, pigmentation inside the eye, increase in pupil size, change in the texture of the eyelashes.
General side effects: eye viral infection, bad taste, irregular or decreased heart rate, increased or decreased blood pressure, cough, voice changes, gastrointestinal discomfort or ulcer, constipation, dry mouth, redness or itching of the skin, rash, hair colour change, loss of eyelashes, musculoskeletal pain, generalised weakness, herpes infection, pain in the throat, problems breathing.
Not known: frequency cannot be estimated from the available data
Effects in the eye: swelling of the back of the eye, eyes appear more inset.
General side effects: depression, anxiety, sensation of false movement, ringing in ears, chest pain, worsening of asthma, diarrhoea, abdominal pain, nausea, itching, abnormal hair growth, joint pain, painful or involuntary urination, increase in prostate cancer marker, very slow or fast heart beat.
In children and adolescents, the most common side effects seen with travoprost are eye redness and growth of eyelashes. Both side effects were observed with a higher incidence in children and adolescents compared to adults.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
HOW TO STORE TRAVOPROST
Keep this medicine out of the sight and reach of children.
Do not use Travoprost after the expiry date which is stated on the bottle and the box after 'Exp’.
The expiry date refers to the last day of the month.
Before opening, keep bottle in overwrap pouch in order to protect from moisture.
After first opening, this medicine does not require any special storage conditions.
Do not use this medicine if you notice that the tamper evident seal has been broken or damaged before you first open it.
You must throw away the bottle 4 weeks after you first opened it, to prevent infections, and use a new bottle. Write down the date you opened it in the space on each bottle label and box.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
©I CONTENTS OF THE PACK AND OTHER INFORMATION
What Travoprost contains
The active substance is travoprost 40 micrograms/ml.
The other ingredients are: benzalkonium chloride, macrogol glycerol hydroxy stearate (cremophor RH40), trometamol, disodium edetate, boric acid (E284), mannitol (E421), sodium hydroxide (for pH adjustment), water for injection.
What Travoprost looks like and the contents of the pack
Travoprost is a liquid (a clear, colourless solution) supplied in a pack containing a 5 ml plastic bottle with a screw cap or in a pack containing one, three or six 5 ml plastic bottles with screw caps. Each bottle contains 2.5 ml solution. Each bottle is placed in a pouch. Not all pack sizes may be marketed.
Marketing Authorisation Holder
TEVA UK Limited, Eastbourne, BN22 9AG, UK
Manufacturers
Pharmathen S.A, Dervenakion 6, Pallini 15351, Attikis, Greece
This leaflet was last revised in 04/2016.
PL 00289/1785
90227-E 300x160 269221.02-GB