Travoprost Sandoz 40 Micrograms/Ml Eye Drops Solution
KAPVORM
PMS ZWART a 100% -20%
August 22, 2014 14:25:48
64926-0
PACKAGE LEAFLET: INFORMATION FOR THE USER
travoprost
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Travoprost is and what it is used for
2. What you need to know before you use Travoprost
3. How to use Travoprost
4. Possible side effects
5. How to store Travoprost
6. Contents of the pack and other information
3
Travoprost eye drops are used to treat high pressure in the eye. This pressure can lead to an illness called glaucoma.
High pressure in the eye: Your eyeballs contain a clear, watery liquid which feeds the inside of the eye. Liquid is always emptying out of the eye, and more liquid is always being produced. If the eye fills up faster than it empties, the pressure inside the eye builds up. If it gets too high, it can damage your sight.
Travoprost is one of a group of medicines for glaucoma called prostaglandin analogues. It works by increasing the outflow of liquid, which lowers the pressure in the eye. It may be used on its own or with other drops e.g. beta-blockers, which also reduce pressure.
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is:
Adults: 1 drop in the eye or eyes, once a day in the evening.
Only use Travoprost in both eyes if your doctor told you to. Take it for as long as your doctor told you to.
Only use Travoprost for dropping in your eyes. Instruction for use:
2 What you need to know before you use Travoprost
Do not use Travoprost
• if you are allergic to travoprost or any of the other ingredients of this medicine (listed in section 6).
Ask your doctor for advice if this applies to you.
Warnings and precautions
Talk to your doctor or pharmacist before using Travoprost.
• Travoprost may increase the length, thickness, colour and/or number of your eyelashes and may cause unusual hair growth on your eyelids.
• Travoprost may change the colour of your iris (the coloured part of your eye). This change may be permanent.
• Travoprost may rarely cause breathlessness or wheezing or increase the symptoms of asthma. If you are concerned about changes in your breathing pattern when using Travoprost advise your doctor as soon as possible.
• Travoprost may be absorbed through the skin and therefore should not be used by women who are pregnant or are attempting to become pregnant. If any of the product comes into contact with the skin then it should be washed off straight away.
Children and adolescents
Travoprost is not to be used by people under 18 years of age.
Other medicines and Travoprost
Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines.
Pregnancy and breast-feeding
Do not use Travoprost if you are pregnant. If you think that you may be pregnant speak with your doctor right away. If you could become pregnant you must use adequate contraception whilst you use Travoprost.
Do not use Travoprost if you are breast-feeding, Travoprost may get into your milk.
Ask your doctor for advice before taking any medicine.
Driving and using machines
You may find that your vision is blurred for a time just after you use Travoprost. Do not drive or use machines until this has worn off.
Travoprost contains
• benzalkonium chloride: This may cause eye irritation. Avoid contact with soft contact lenses. Remove contact lenses prior to application and wait at least 15 minutes before reinsertion. Benzalkonium chloride is known to discolour soft contact lenses.
• macrogolglycerol hydroxystearate 40: This may cause skin reactions.
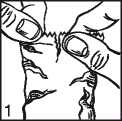
• Immediately before using a bottle for the first time, tear-off the overwrap pouch take it out (picture 1) and write the date of opening on the label in the space provided.
• Get the Travoprost bottle and a mirror.
• Wash your hands.
• Twist off the cap.
• Hold the bottle, pointing down, between your thumb and fingers.
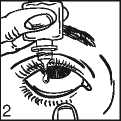
• Tilt your head back. Pull down your eyelid with a clean finger, until there is a ‘pocket’ between the eyelid and your eye. The drop will go in here (picture 2).
• Bring the bottle tip close to the eye. Use the mirror if it helps.
• Don’t touch your eye or eyelid, surrounding areas or other surfaces with the dropper. It could infect the drops.
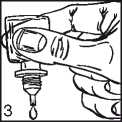
• Gently squeeze the bottle to release one drop of Travoprost at a time (picture 3).
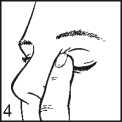
• After using Travoprost, press a finger into the corner of your eye, by the nose (picture 4).
This helps to stop Travoprost getting into the rest of the body.
• If you take drops in both eyes, repeat the steps for your other eye.
• Close the bottle cap firmly immediately after use.
• Only use one bottle at a time. Do not open the pouch until you need to use the bottle.
If a drop misses your eye, try again.
If you use more Travoprost than you should
Rinse it all out with warm water. Don’t put in any more drops until it’s time for your next regular dose.
If you forget to use Travoprost
Continue with the next dose as planned. Do not use a double dose to make up for a forgotten dose. The dose should not exceed one drop in the affected eye(s) daily.
If you stop using Travoprost
Without speaking to your doctor, the pressure in your eye will not be controlled which could lead to loss of sight.
If you are using other eye drops, leave at least 5 minutes between putting in Travoprost and the other drops.
If you have any further questions on the use of this medicine ask a doctor or pharmacist.
Continued on the next page >>
InDesign CS4
|
V1 Alcori BELGIUM |
64926-0 -DRAFT- |
|
140 x 504 / 140 x 22 mm |
Travoprost 0.004% 2.5 ml / 3 x 2.5 ml |
|
recto-verso |
GB |
|
AANTAL KLEUREN: 1 |
HVR 22-06-2014 |
|
§ |
Local | ||
|
5 |
Graphics | ||
|
gj CD | |||
|
5 O cs ot> o o O LU CO |
Approval |
Affiliates |
confirms that this proof contains an accurate translation of the English Corporate Standard text, and is in compliance with the registered information and the legal rules. Please also carefully check: local barcodes if any, formula, shelflife if mentioned, storage conditions and trademarks. This proof is approved Signature and date: □ as is. Q as is, waiting for M.O.H. approval. , . . (do not order component vet) Customer reauests □ new proof. |
|
Local |
Final release: □ as is. □ new proof. | ||
|
Graphics |
KAPVORM
PMS ZWART a 100% -20%
August 22, 2014 14:25:48
Like all medicines, this medicine can cause side effects although not everybody gets them.
You can usually carry on taking the drops, unless the effects are serious. If you’re worried, talk to a doctor or pharmacist. Do not stop taking Travoprost without speaking to your doctor.
Very common side effects, affects more than 1 per 10 users
Redness of the eye, changes in the colour of the iris (coloured part of the eye; this may affect up to 23 % of the patients)
Common side effects, affects 1 to 10 per
100 users
Effects in the eye:
• inflammation inside the eye
• eye pain or swelling
• eye irritation
• eye discharge
• sensitivity to light
• blurred
• reduced or abnormal vision
• dry eye, itchy eye
• increased tear production
• abnormal or decreased eye sensation
• eyelid abnormality, irritation, itching, redness, pain, swelling or crusting
• discolouration of the eyelashes, increased or decreased growth or number of eyelashes
Effects in the body:
• headache
• skin darkening around the eyes
Uncommon side effects, affects 1 to 10 per
1000 users
Effects in the eye:
• inflammation or infection of the conjunctiva or cornea
• halo vision
• corneal disorder
• eye allergy
• tired eyes
• increase in pupil size
Effects in the body:
• asthma, shortness of breath
• increased or decreased blood pressure
• irregular, increased, or decreased heart rate
• dizziness
• viral infection
• cough
• generalised weakness
• increased allergic symptoms
• throat irritation
• stuffy nose
• voice changes
• gastrointestinal discomfort or ulcer
• constipation
• redness or itching
• shoulder pain
• bad taste
• dry mouth
Frequency unknown, according to the available data:
Effects in the eye:
• inflammation of the back of the eye
• sunken eyes
Effects in the body:
• worsening of asthma
• ringing in ears
• increased prostate antigen
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme (www.mhra.gov.uk/yellowcard). By reporting side effects you can help provide more information on the safety of this medicine.
What Travoprost contains
• The active substance is travoprost. Each ml of solution contains 40 micrograms travoprost.
• The other ingredients are: Benzalkonium chloride solution, macrogolglycerol hydroxystearate 40, trometamol, disodium edetate, boric acid, mannitol and purified water. Tiny amounts of hydrochloric acid or sodium hydroxide are added to keep acidity levels (pH levels) normal.
What Travoprost looks like and contents of the pack
Travoprost is a clear, colourless eye drop solution, supplied in a pack containing 1,3 or 6 plastic dropper container(s) of 2.5 ml, each with a screw cap. Each dropper container is placed in a pouch.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Sandoz Ltd.
Frimley Business Park,
Frimley, Camberley,
Surrey,
GU16 7SR United Kingdom
Manufacturer:
Alcon-Couvreur N.V Rijksweg 14, B-2870 Puurs Belgium
Or
Alcon Cusf S.A
Camil Fabra 58, 08320 El Masnou, Barcelona Spain
Or
Lek Pharmaceuticals d.d.
Verovskova 57, 1526 Ljubljana Slovenia
Or
Salutas Pharma GmbH Otto-von-Guericke-Allee 1,39179 Barleben Germany
Or
Aeropharm GmbH
Francois-mitterrand-allee 1,07407 Rudolstadt Germany
This leaflet was last revised in 08/2014.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, the pouch and the bottle label after ‘EXP’. The expiry date refers to the last day of that month.
This medicine does not require any special storage conditions.
You must throw away the bottle 4 weeks after you first opened it, to prevent infections, and use a new bottle. Write down the date you opened it in the space on each bottle label and box.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
Sandoz and ASANDOZ are registered trademarks of Novartis.
05-2014 64926-0

InDesign CS4
|
V1 Alcon BELGIUM |
64926-0 -DRAFT- |
|
140 x 504 / 140 x 22 mm |
Travoprost 0.004% 2.5 ml / 3 x 2.5 ml |
|
recto-verso |
GB |
|
AANTAL KLEUREN: 1 |
HVR 22-06-2014 |
|
§ |
Local | ||
|
5 |
Graphics | ||
|
gj CD | |||
|
5 O cs od O O O LU CO |
Approval |
Affiliates |
confirms that this proof contains an accurate translation of the English Corporate Standard text, and is in compliance with the registered information and the legal rules. Please also carefully check: local barcodes if any, formula, shelflife if mentioned, storage conditions and trademarks. This proof is approved Signature and date: □ as is. Q as is, waiting for M.O.H. approval. , . . (do not order component vet) Customer reauests □ new proof. |
|
Local |
Final release: □ as is. □ new proof. | ||
|
Graphics |