Travoprost Teva 40 Micrograms/Ml Eye Drops Solution
teva UK Ref: 231-30-70414-A LEA TRAVOPROST 40 MCG/ML 2.5 ML TUK <AET Version: 4 17 February 2015
teva UK Ref: 231-30-70414-A LEA TRAVOPROST 40 MCG/ML 2.5 ML TUK <AET Version: 4 17 February 2015
12345
TRAVOPROST 40 micrograms/ml EYE DROPS, SOLUTION
Package leaflet: Information for the user

Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Travoprost is and what it is used for
2. What you need to know before you use Travoprost
3. How to use Travoprost
4. Possible side effects
5. How to store Travoprost
6. Contents of the pack and other information
^ What Travoprost is and what it is used for
Travoprost contains travoprost,
one of a group of medicines called prostaglandin analogues. It works by reducing the pressure in the eye. It may be used on its own or with other drops e.g. beta-blockers, which also reduce pressure.
Travoprost is used to reduce high pressure in the eye in adults. This pressure can lead to an illness called glaucoma.
What you need to know before you use Travoprost
Do not use Travoprost
• if you are allergic to travoprost or any of the other ingredients of this medicine (listed in section 6).
Ask your doctor for advice if this applies to you.
Warnings and precautions
Talk to your doctor or, pharmacist before using Travoprost
• Travoprost may increase the
length, thickness, colour and/or number of your eyelashes. Changes in the eyelids including unusual hair growth or in the tissues around the eye have also been observed
• Travoprost may change the colour of your iris (the coloured part of your eye). This change may be permanent. A change in the colour of the skin around the eye may also occur
• if you have had cataract surgery, talk to your doctor before you use Travoprost
• if you have current or previous history of an eye inflammation (iritis and uveitis), talk to your doctor before you use Travoprost
• Travoprost may rarely cause breathlessness or wheezing or increase the symptoms of asthma. If you are concerned about changes in your breathing pattern when using Travoprost advise your doctor as soon as possible
• travoprost may be absorbed through the skin. If any of the
medicinal product comes into contact with the skin, it should be washed off straight away.
This is especially important in women who are pregnant or are attempting to become pregnant
• if you wear soft contact lenses, do not use the drops with your lenses in. After using the drops wait 15 minutes before putting your lenses back in.
Children and adolescents
Use of Travoprost is not recommended to those under 18 years of age.
Other medicines and Travoprost
Tell your doctor or pharmacist if you are taking, have recently taken or used any other medicines.
Pregnancy, breast-feeding and fertility
Do not use Travoprost if you are pregnant. If you think that you may be pregnant speak with your doctor right away. If you could become pregnant you must use adequate contraception whilst you use Travoprost.
Do not use Travoprost if you are breast-feeding, Travoprost may get into your milk.
Ask your doctor for advice before taking any medicine.
Driving and using machines
You may find that your vision is blurred for a time just after you use Travoprost. Do not drive or use machines until this has worn off.
Travoprost contains macrogolglycerol hydroxystearate and propylene glycol which may cause skin reactions and irritation.
How to use Travoprost
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is
One drop in the affected eye or eyes, once a day - in the evening.
Only use Travoprost in both eyes if your doctor told you to. Use it for as long as your doctor told you to.
Only use Travoprost for dropping in your eyes.

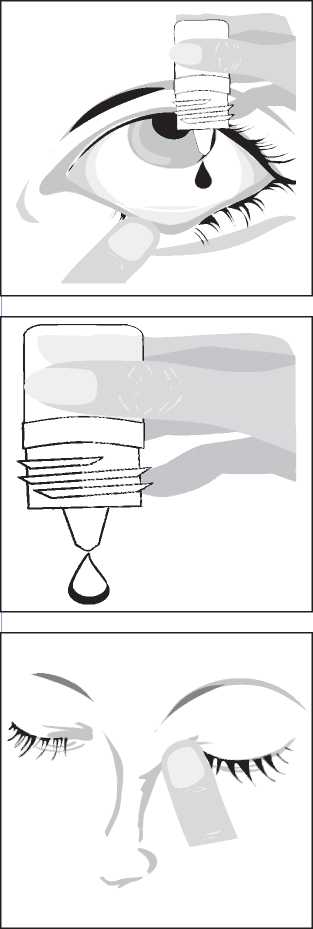
• immediately before using a bottle for the first time, write the date of opening on the carton in the space provided
• wash your hands
• twist off the cap
• after cap is removed, if tamper evident snap collar is loose, remove before using the product
• hold the bottle, pointing down, between your thumb and fingers
• tilt your head back. Pull down your eyelid with a clean finger, until there is a 'pocket' between the eyelid and your eye. The drop will go in here
• bring the bottle tip close to the eye. Use a mirror if it helps
• do not touch your eye or eyelid, surrounding areas or other surfaces with the dropper. It could infect the drops
• gently squeeze the bottle to release one drop of Travoprost at a time
• after using Travoprost, keep the eyelid closed, apply gentle pressure by pressing a finger into the corner of your eye, by the nose for at least 1 minute. This helps to stop Travoprost getting into the rest of the body
• if you use drops in both eyes, repeat the steps for your other eye
• close the bottle cap firmly immediately after use
• only use one bottle at a time.
If a drop misses your eye, try again.
If you are using other eye preparations such as eye drop or eye ointment, wait for at least 5 minutes between putting in Travoprost and the other eye preparations.
12345
|
TEVA UK Ref: 231-30-70414-A LEA TRAVOPROST 40 MCG/ML 2.5 ML TUK <AET |
Version: 4 17 February 2015 | ||
3
If you use more Travoprost than you should
Rinse all the medicine out with warm water. Don't put in any more drops until it's time for your next regular dose.
If you forget to use Travoprost
Continue with the next dose as planned. Do not use a double dose to make up for a forgotten dose. Never use more than one drop in the affected eye(s) in a single day.
If you stop using Travoprost
Do not stop using Travoprost without first speaking to your doctor, the pressure in your eye will not be controlled which could lead to loss of sight.
If you have any further questions on the use of this medicine ask your doctor or pharmacist.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You can usually carry on using the drops, unless the side effects are serious. If you're worried, talk to a doctor or pharmacist. Do not stop taking Travoprost without speaking to your doctor.
The following side effects have been seen with travoprost containing eye drops
Very common side effects: may affect more than 1 in 10 people
• Effects in the eye: eye redness.
Common side effects: may affect up to 1 in 10 people
• Effects in the eye: changes in the colour of the iris (coloured part of the eye), eye pain, eye discomfort, dry eye, itchy eye, eye irritation.
Uncommon side effects: may affect up to 1 in 100 people
• Effects in the eye: corneal disorder, eye inflammation, iris inflammation, inflammation inside the eye, eye surface inflammation with/out surface damage, sensitivity to light, eye discharge, eyelid inflammation, eyelid redness, swelling around the eye, eyelid itching, reduced vision, blurred vision, increased tear production, infection or inflammation of the conjunctiva (conjunctivitis), abnormal turning outward of the lower eyelid, clouding of the eye, eyelid crusting, growth of eyelashes, discolouration of the eyelashes, tired eyes
• General side effects: increased allergic symptoms, headache, dizziness, irregular heart beat, shortness of breath, asthma, stuffy nose, throat irritation, darkening of skin around the eye (s), skin darkening, abnormal hair texture, excessive hair growth.
Rare: may affect up to 1 in 1,000 people
• Effects in the eye: perception of flashing lights, eczema of the eyelids, eye swelling, halo vision, decreased eye sensation, inflammation of the glands of the eyelids, pigmentation inside the eye, increase in pupil size, change in the texture of the eyelashes
• General side effects: eye viral infection, bad taste, irregular or decreased heart rate, increased or decreased blood pressure, cough, voice changes, gastrointestinal discomfort or ulcer, constipation, dry mouth, redness or itching of the skin, rash, hair colour change, loss of eyelashes, musculoskeletal pain, generalised weakness.
Not known: frequency cannot be estimated from the available data
• Effects in the eye: inflammation of the back of the eye, eyes appear more inset
• General side effects: depression, anxiety, sensation of false movement, ringing in ears, chest pain, worsening of asthma, diarrhoea, abdominal pain, nausea, itching, abnormal hair growth, joint pain, painful or involuntary urination, increase in prostate cancer marker.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Yellow Card Scheme at:
By reporting side effects you can help provide more information on the safety of this medicine.
How to store Travoprost
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the bottle and the box after 'Exp'. The expiry date refers to the last day of the month.
This medicine does not require any special temperature storage conditions. Keep the bottle in the outer carton in order to protect from light.
You must throw away the bottle 4 weeks after you first opened it, to
prevent infections, and use a new bottle. Write down the date you opened it in the space on the carton box.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
Contents of the pack and other information
What Travoprost contains
• The active substance is travoprost 40 micrograms/ml The other ingredients are: Polyquaternium-1, macrogolglycerol hydroxystearate 40, propylene glycol (E1520), sodium chloride, boric acid (E284), mannitol (E421) and purified water. Sodium hydroxide or hydrochloric acid for pH adjustment.
What Travoprost looks like and contents of the pack
Travoprost is a clear, colourless solution, practically free of particles in a pack containing either 1, 3 or 6 plastic bottles each with a screw cap. Each bottle contains 2.5 ml solution.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
TEVA UK Limited, Eastbourne, BN22 9AG, UK
Manufacturer
S.C. Rompharm Company S.R.L., Eroilor Street, no. 1A, Otopeni, Ilfov., Romania, 075100
This leaflet was last revised in 02/2015
PL 00289/1861
70414-A
150x640
TEUZD
TEVA UK LIMITED