Treosulfan Injection
Treosulfan Injection • 80310-VBGB • BA • 02.15 • Pharma-Code: 533
Format: 296 x 150 mm • HKS 44 • Corrective action: KV01_jem_19.02.15
PACKAGE LEAFLET: INFORMATION FOR THE USER
Treosulfan Injection
Treosulfan
Read all of this leaflet carefully before you are
given this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you.
Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
^ In this leaflet:
1. What Treosulfan Injection is and what it is used for
2. Before you are given Treosulfan Injection
3. How you are given Treosulfan Injection
4. Possible side effects
5. How to store Treosulfan Injection
6. Further information
1. WHAT TREOSULFAN INJECTION IS AND WHAT IT IS USED FOR
Treosulfan belongs to the group of anticancer medicines called bifunctional alkylating agents. These agents inhibit tumour growth.
Treosulfan Injection has been prescribed by your doctor for the treatment of all types of ovarian cancer or certain types of cancer which do not respond to usually given kind of therapy.
2. BEFORE YOU ARE GIVEN TREOSULFAN INJECTION
You are not given Treosulfan Injection, if you
• are allergic (hypersensitive) to Treosulfan.
• do not have enough blood cells (severe bone marrow depression).
You will have blood tests to check that you have enough blood cells to receive Treosulfan Injection before each administration.
Take special care with Treosulfan Injection, if you
• experience reduction in blood cells as this may become worse with ongoing treatment. Blood tests will be performed at shorter intervals starting with the third course of treatment.
This is especially important if combined with other forms of therapy that suppress the bone marrow function such as radiotherapy.
• develop inflammation of the lungs which causes shortness of breath (allergic alveolitis or pulmonary fibrosis); treatment with treosulfan should then be stopped.
Be also aware that:
• the risk of getting different types of infections is increased.
• treatment with cytostatics may increase the risk of generalised infection after some vaccinations. Therefore you should not receive vaccination with live vaccines.
• because of the possible development of bladder inflammation with pain, more frequent or urgent urination with or without bloody urine (haemorrhagic cystitis) you are advised to drink more fluids than usual for up to 24 hours after your treosulfan infusion.
Taking other medicines
Please tell your doctor or pharmacist if you are
taking or have recently taken any other medicines,
including medicines obtained without a prescription.
It is not advisable to use any medical treatment without telling your doctor as there may be interactions between treosulfan and other medicines.
Pregnancy and breast-feeding
Do not take treosulfan if you are pregnant or planning to become pregnant. You must use effective contraception during therapy, e.g. birth control pill.
Do not breast-feed during treatment with treosulfan.
If you are thinking of becoming pregnant or breast-feeding discuss it with your doctor first.
Driving and using machines
Your ability to drive or operate machines may be influenced because of nausea and vomiting. If you are affected in this way do not drive or operate machinery.
3. HOW YOU ARE GIVEN TREOSULFAN INJECTION
Treosulfan will be given to you
• by an injection into your vein (intravenous injection),
• by an injection into your abdomen (intraperitoneal injection), or
• by a drip given into a vein (intravenous infusion).
Doses up to 1.5 g/m2 (into abdomen) or 3 g/m2 (into vein) will be given as an injection. Larger doses will be administered as a drip given into a vein (intravenous infusion) at a rate of 3 g/m2 every 5 - 10 minutes (8 g/m2 as a 30 minutes infusion).
Your doctor will calculate your treosulfan dose on the basis of your blood counts measured. Your doctor reduces the dose if other cytotoxic medicines or radiotherapy have also been given. The dose you are given also depends on your size and varies with your body surface area. Technically, this is measured in square metres (m2), but actually is worked out from your height and weight.
During the course of treosulfan therapy the injections will be given usually every 1 to 3 weeks.
Your doctor may change the dose and frequency of dosing depending on your blood tests, your general condition, further therapies and your response to treosulfan. If you have any questions about your treatment, ask your doctor, nurse or hospital pharmacist.
If you experience pain at the site of injection please tell your doctor or nurse immediately.
Children
This medicine is not recommended for use in children.
If you are given more Treosulfan than you should
If too much medication has been given to you, your doctor may give you a blood transfusion and will undertake other measures if necessary.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, treosulfan can cause side effects, although not everybody gets them.
Contact your doctor straight away if you notice any of the following:
• Allergic reactions: if you develop itching, rash, swelling of the face, lips, tongue and/or throat, which may cause difficulties in swallowing or breathing, drop of blood pressure.
• Fever or infection: if you have a body temperature of 38 °C or higher, sweating or other signs of infection (since you might have fewer white blood cells than normal).
• Weakness, becoming easily breathless or if you look pale (since you might have fewer red blood cells than normal).
• Bleedings from gums, mouth or nose, unexpected bruising (since you might have fewer platelets than normal).
• Difficulty in breathing (since you might have an allergic reaction, inflammation or infection of the lung).
Very common (affect more than 1 in 10 patients):
• Reduction in white blood cells (which make infections more likely), platelets (which can cause bruising and bleeding from gums, mouth and nose) and red blood cells (which can make the skin pale and cause weakness or breathlessness) - hence the need for regular blood tests.
• Stomach upsets including nausea (feeling sick) with or without vomiting (being sick).
• Mild loss of hair. After your treatment, normal hair growth should return.
• Bronze discolouration of the skin.
Uncommon (affect 1 to 10 in 1,000 patients):
• Different types of blood cancer (after long-term treatment).
Not known (frequency cannot be estimated from
the available data):
• Allergic reactions (e.g. itching, rash, swelling of the face, lips, tongue and/or throat with difficulties in swallowing or breathing, drop of blood pressure).
• Severe reduction of different blood cells at the same time which can cause weakness, bruising or make infections more likely (pancytopenia).
The following side effects have also been reported:
• Addison’s disease, a condition where the adrenal glands do not work properly, leading to bronzed skin, stomach upset, low blood pressure (feeling faint) and a general feeling of weakness.
• Sweating, trembling and hunger as a result of the decreased amount of glucose in your blood (hypoglycaemia).
• Pins and needles and a feeling of numbness (paraesthesia).
• Weakening of the heart muscle caused by a structural change (cardiomyopathy).
• Difficulty in breathing (inflammation and scarring of the lungs and infection of the lungs).
• Urticaria or hives, an itchy rash; inflammation of the skin with or without scale formation (scleroderma and psoriasis).
• Inflammation of the bladder with pain, more frequent and urgent urination and with or without bloody urine (haemorrhagic cystitis).
• Feeling of getting ill (flu-like complaints).
• Painful local redness and swelling at the injection site (in case of leakage of treosulfan solution into the surrounding tissue).
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE TREOSULFAN
Keep out of the reach and sight of children.
Do not use Treosulfan Injection after the expiry date which is stated on the vial after EXP. The expiry date refers to the last day of that month.
6. FURTHER INFORMATION
What Treosulfan Injection contains
The active substance is treosulfan.
What Treosulfan Injection looks like and contents of the pack
Each glass vial contains 1 g or 5 g of treosulfan.
The dry-powder in its vial is mixed with water for injection to form a solution before it is given to you.
Treosulfan vials are packed in boxes, each containing 5 vials.
Marketing Authorisation Holder and Manufacturer
medac
Gesellschaft fur klinische Spezialpraparate mbH Theaterstr. 6 22880 Wedel Germany
This leaflet was last approved in 01/2015.
Full information is available on request from
medac
Gesellschaft fur klinische Spezialpraparate mbH Scion House
Stirling University Innovation Park Stirling FK9 4NF Tel.: 01786/ 458 086 Fax: 01786/ 458 032
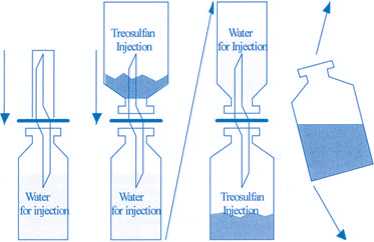
Instructions for reconstitution of Treosulfan Injection
To avoid solubility problems during reconstitution the following aspects should be regarded.
1. The solvent, water for injection, is warmed to 25 - 30 °C (not higher!) by using a water bath.
2. The treosulfan is carefully removed from the inner surface of the infusion bottle by shaking.
This procedure is very important, because moistening of powder that sticks to the surface results in caking. In case caking occurs the bottle has to be shaken long and vigorously.
3. One side of the double sided cannula is put into the rubber stopper of the water bottle.
The treosulfan bottle is then put on the other end of the cannula with the bottom on top.
The whole construction is converted and the water let run into the lower bottle while the bottle is shaken gently.
Following these instructions, the whole reconstitution procedure should take no longer than 2 minutes.
12 3 4
80310-VBGB
BA