Trinovum Oral Contraceptive Tablets
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Trinovum Oral Contraceptive Tablets
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
7 white tablets Norethisterone 0.5 mg
Ethinylestradiol 0.035 mg
7 pale peach coloured tablets Norethisterone 0.75 mg
Ethinylestradiol 0.035 mg
7 peach coloured tablets Norethisterone 1.0 mg
Ethinylestradiol 0.035 mg
For the full list of excipients, see section 6.1
3 PHARMACEUTICAL FORM
Tablets
White tablets - small round, white tablet engraved with “C 535" on both faces
Pale peach tablets -small round pale-peach coloured tablet engraved with "C 735" on both faces.
Peach tablets - small round, peach-coloured tablets engraved with “C 135” on both faces.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Oral contraception and the recognised indications for such oestrogen/progestogen combinations.
4.2 Posology and method of administration
For oral administration.
4.2.1 Adults
How to take Trinovum:
One tablet is taken daily at the same time (preferably in the evening) without interruption for 21 days, followed by a break of 7 tablet-free days. (A white tablet is taken every day for 7 days, then a light peach coloured tablet is taken every day for 7 days, then a peach coloured tablet every day for 7 days, followed by 7 tablet-free days.) Each subsequent strip is started after the 7 tablet-free days have elapsed. Additional contraceptive precautions are not then required. During the tablet-free period, bleeding can be expected, usually beginning 2 to 4 days after the last tablet.
Starting treatment:
It is preferable that tablet intake from the first pack is started up to and including day 5 of menstruation in which case no extra contraceptive precautions are necessary.
Trinovum can be started at any other time, if pregnancy can reasonably be excluded. In this case additional contraceptive precautions must be taken for the first 7 days of tablet taking.
Switching from another contraceptive
Hormonal methods: Trinovum can be started immediately if the patient has been using the current hormonal method consistently and correctly, or if pregnancy can reasonably be excluded. There is no need to wait for the next menstruation. Additional contraceptive precautions are not required.
Non-hormonal methods: If Trinovum is started after the first 5 days of menstruation, additional contraceptive precautions are required for the next 7 days.
Post-partum administration
Following a vaginal delivery, oral contraceptive administration to non-breastfeeding mothers can be started 21 days post-partum provided the patient is fully ambulant and there are no puerperal complications. No additional contraceptive precautions are required. If post-partum administration begins more than 21 days after delivery, additional contraceptive precautions are required for the first 7 days of pill-taking.
If intercourse has taken place post-partum, oral contraceptive use should be delayed until the first day of the first menstrual period.
For information on breast-feeding mothers, see sections 4.3, 4.4 and 4.6.
Use after Abortion or Miscarriage
After an abortion or miscarriage that occurs prior to 24 weeks gestation, oral contraceptives can be started immediately. An additional method of contraception is not needed.
After an induced or spontaneous abortion that occurs at or after 24 weeks gestation, hormonal contraceptives may be started either on Day 21 post-abortion or on the first day of the first spontaneous menstruation, whichever comes first. No additional contraceptive precautions are required.
To skip a period
To skip a period, a new pack of Trinovum should be started on the day after finishing the current pack (the patient skips the tablet-free days). Tablet-taking should be continued in the usual way.
During the use of the second pack she may experience slight spotting or breakthrough bleeding but contraceptive protection will not be diminished provided there are no tablet omissions.
The next pack of Trinovum is started after the usual 7 tablet-free days, regardless of whether the period has completely finished or not.
Reduced reliability
When Trinovum is taken according to the directions for use, the occurrence of pregnancy is highly unlikely. However, the reliability of oral contraceptives may be reduced under the following circumstances:
(i) Missed tablets
If the patient forgets to take one tablet or if a new strip is started one day late, she should take it as soon as she remembers and take the next tablet at the normal time. This may mean that two tablets are taken in one day. No additional contraceptive precautions are required.
If more than one tablet is missed or if a new strip is started more than one day late, she should take the last missed tablet as soon as she remembers but leave the other missed tablets in the strip. She should continue to take the rest of the strip as usual but must use extra precautions (e.g. condom, diaphragm, plus spermicide) for the next 7 days.
If the tablets are missed:
• In week 1 If unprotected sex has taken place, the use of emergency contraception should be considered. The usual 7-day break can be left before starting the next strip.
• In week 2 The usual 7-day break can be left before starting the next strip.
• In week 3 When the strip is finished the next strip should be started the next day without a break. If withdrawal bleeding does not occur at the end of the second strip, a pregnancy test should be performed.
(ii) Vomiting or diarrhoea
If a patient vomits within two hours of taking a tablet she should take another tablet from a spare strip.
If severe vomiting or diarrhoea continues for more than 1 day, she should follow the procedure for missed tablets (and continue taking the tablets if she can).
4.2.2 Elderly
Use of this product is not indicated in post-menopausal women.
4.2.3 Children
Use of this product before menarche is not indicated.
4.3 Contraindications
- Breast-feeding mothers less than 6 weeks post-partum
- Venous thrombo-embolism (VTE) requiring concurrent anticoagulant therapy, personal history of confirmed VTE or known thrombogenic mutations.
- Major surgery with prolonged immobilisation.
- Moderate to severe hypertension (systolic >160 mm Hg or diastolic >95 mm Hg), current or history of ischaemic heart disease, stroke, peripheral vascular disease or presence of multiple risk factors for arterial disease (see section 4.4).
- Complicated valvular and congenital heart disease (e.g. with pulmonary hypertension, artrial fibrillation, history of subacute bacterial endocarditis)
- Migraine with focal aura.
- Diabetes with nephropathy/retinopathy/neuropathy or other vascular involvement or > 20 years’ duration.
- Smoking 15 or more cigarettes per day in patients aged 35 years or more.
- Acute or chronic liver disease, including hepatitis (viral or non-viral) or severe cirrhosis, or a history of these conditions until at least 3 months after abnormal liver function tests have returned to normal; hepatic adenomas or carcinomas.
- Known or suspected carcinoma of the breast.
- Raynaud’s disease, with Systemic Lupus Erythematosus (SLE) if lupus anticoagulant is present.
- Hypersensitivity to the active substances (norethisterone or ethinylestradiol) or to any of the excipients listed in section 6.1.
Should any of these conditions occur for the first time during use of Binovum, the tablets should be discontinued immediately.
4.4 Special warnings and precautions for use
Assessment of women prior to starting oral contraceptives (and at regular intervals thereafter) should include a personal and family medical history of each woman. Physical examination should be guided by this and by the contraindications (Section 4.3) and warnings for this product. The frequency and nature of these assessments should be based upon relevant guidelines and should be adapted to the individual woman, but should include measurement of blood pressure and, if judged appropriate by the clinician, breast, abdominal and pelvic examination including cervical cytology.
Exclude likelihood of pregnancy before starting treatment.
Undiagnosed vaginal bleeding should be investigated further.
Oral contraceptives DO NOT protect against HIV infections (AIDS) or any other sexually transmitted disease.
Conditions requiring supervision:
The theoretical or proven risks usually outweigh the advantages of using Combined Oral Contraceptives (COCs) in the conditions listed below. Consequently the decision to prescribe the COC must be made with specialist clinical judgement and in consultation with the individual patient. If any of these conditions appears for the first time, or is aggravated, whilst the patient is taking Trinovum, consideration should be given to discontinuing its use.
- Non-breast-feeding mothers less than 21 days post-partum.
- Breast-feeding mothers 6 weeks to 6 months post-partum.
- Increased risk of venous thrombo-embolic disorders (See “Circulatory disorders” below).
- Presence of multiple risk factors for arterial disease (See “Circulatory disorders” below).
- Adequately controlled hypertension (persistently elevated baseline systolic values 140-159 mm Hg or diastolic values 90-94 mm Hg)
- Obesity (BMI > 35 kg/m2)
- Past history (> 5 years ago) of migraine with aura. The onset or exacerbation of migraine or development of headache with a new pattern which is recurrent, persistent or severe requires discontinuation of oral contraceptives and evaluation of the cause.
- History of cholestasis (related to COCs), current or medically treated gall bladder disease, porphyria.
- History of breast cancer, 5 years disease-free.
- Diabetes mellitus with mild nephropathy, neuropathy or retinopathy.
Circulatory disorders
Venous Thrombo-Embolism (VTE)
An increased risk of venous thrombo-embolic disease (VTE) associated with the use of oral contraceptives is well established but is smaller than that associated with pregnancy, which has been estimated at 60 cases per 100,000 pregnancies. Some epidemiological studies have reported a greater risk of VTE for women using combined oral contraceptives containing desogestrel or gestodene (the so-called ‘third generation’ pills) than for women using pills containing levonorgestrel or norethisterone (the so-called ‘second generation’ pills).
The spontaneous incidence of VTE in healthy non-pregnant women (not taking any oral contraceptive) is about 5 cases per 100,000 per year. The incidence in users of second generation pills is about 15 per 100,000 women per year of use. The incidence in users of third generation pills is about 25 cases per 100,000 women per year of use; this excess incidence has not been satisfactorily explained by bias or confounding. The level of all of these risks of VTE increases with age and is likely to be further increased in women with other known risk factors for VTE such as obesity. The excess risk of VTE is highest during the first year a woman ever uses a combined oral contraceptive. There is also some evidence that the risk is increased when a Combined Hormonal Contraceptive is re-started after a break in use of 4 weeks or more.
The risk of venous thrombo-embolism increases with
- Increasing age
- Family history (VTE in first degree relative less than 45 years of age)
- Obesity (BMI > 30 kg/m2)
- Prolonged immobilisation, major surgery, any surgery to the legs, major trauma. Hormonal contraception should be discontinued 4-6 weeks before elective surgery and not recommenced until two weeks after complete remobilisation. In case of emergency surgery, thrombotic prophylaxis is usually indicated e.g. with subcutaneous heparin.
There is no consensus about the role of varicose veins in VTE.
Symptoms of venous thrombotic or thrombo-embolic events can include:
• unusual unilateral leg pain and/or redness and swelling, typically of the calf
• sudden breathlessness or onset of coughing
• sudden partial or complete loss of vision.
Arterial thrombo-embolism
Epidemiological studies have also associated the use of COCs with an increased risk for arterial thrombo-embolism (e.g. myocardial infarction, transient ischaemic attack or stroke). This increased risk is likely to be extremely small in women who do not smoke and who do not have other risk factors of arterial thrombo-embolic complications (see below).
The risk of arterial thrombo-embolic complications increases with:
- Increasing age
- Smoking. The risk increases with age and with heavy smoking and is more marked in women over 35 years of age. Women who use oral contraceptives should be strongly advised not to smoke.
- Obesity (BMI > 30 kg/m2)
- Hyperlipidaemia
- Hypertension or a history of hypertension
- Valvular heart disease
- Atrial fibrillation
- Family history of arterial thrombo-embolic complications
- Diabetes mellitus
- Sickle cell haemoglobinopathy.
Symptoms of an arterial or cerebrovascular event can include:
• sudden severe pain in the chest, whether or not it radiates to the left arm
• any unusual, severe, prolonged headache, especially if it occurs for the first time or gets progressively worse
• diplopia or sudden partial or complete loss of vision
• slurred speech or aphasia
• weakness or very marked numbness suddenly affecting one side or one part of the body
• collapse with or without focal seizure, motor disturbances, vertigo.
Hepatic adenomas
Malignant hepatic tumours have been reported on rare occasions in long-term users of oral contraceptives. Benign hepatic tumours have also been associated with oral contraceptive usage. A hepatic tumour should be considered in the differential diagnosis when upper abdominal pain, enlarged liver or signs of intra-abdominal haemorrhage occur. In isolated cases, life-threatening intra-abdominal haemorrhage may occur.
Breast cancer
A meta-analysis from 54 epidemiological studies reported that there is a slightly increased relative risk (RR = 1.24) of having breast cancer diagnosed in women who are currently using COCs. The observed pattern of increased risk may be due to an earlier diagnosis of breast cancer in COC users, the biological effects of COCs or a combination of both. The additional breast cancers diagnosed in current users of COCs or in women who have used COCs in the last 10 years are more likely to be localised to the breast than those in women who never used COCs.
Breast cancer is rare among women under 40 years of age whether or not they take COCs. Whilst this background risk increases with age, the excess number of breast cancer diagnoses in current and recent COC users is small in relation to the overall risk of breast cancer (see bar chart).
The most important risk factor for breast cancer in COC users is the age women discontinue the COC; the older the age at stopping, the more breast cancers are diagnosed. Duration of use is less important and the excess risk gradually disappears during the course of the 10 years after stopping COC use such that by 10 years there appears to be no excess.
The possible increase in risk of breast cancer should be discussed with the user and weighed against the benefits of COCs taking into account the evidence that they offer substantial protection against the risk of developing certain other cancers (e.g. ovarian and endometrial cancer).
Estimated cumulative numbers of breast cancers per 10,000 women diagnosed in 5 years of use and up to 10 years after stopping COCs, compared with numbers of breast cancers diagnosed in 10,000 women who had never used COCs
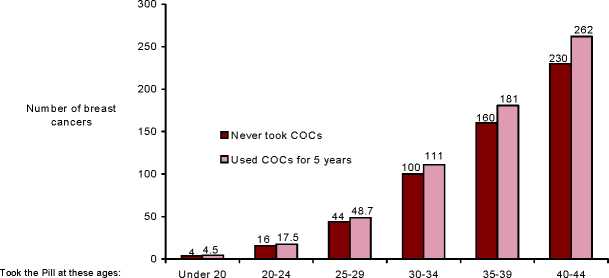
Cancers found up to the age of: 30 35 40 45 50 55
Cervical cancer
The most important risk factor for cervical cancer is persistent Human Papilloma Virus (HPV) infection. Some epidemiological studies have indicated that long-term use of COCs may further contribute to this increased risk but there continues to be controversy about the extent to which this finding is attributable to confounding effects, e.g. cervical screening and sexual behaviour including use of barrier contraceptives.
There is some theoretical concern that COCs enhance progression of Cervical Intraepithelial Neoplasia (CIN) to invasive disease. For women with diagnosed cervical cancer, COCs may be used whilst awaiting treatment.
Other tumours
Numerous epidemiological studies have been reported on the risk of ovarian and endometrial cancer in women using COCs. The evidence is clear that COCs offer substantial protection against both ovarian and endometrial cancer.
Bleeding irregularities
Breakthrough bleeding, spotting and/or absence of withdrawal flow may be encountered in patients on oral contraceptives, especially during the first three months of use.
If bleeding irregularities persist beyond three cycles or occur after previously regular cycles, non-hormonal causes should be considered and adequate diagnostic measures are indicated to exclude malignancy or pregnancy.
Some women may experience post-pill amenorrhoea or oligomenorrhoea, especially when such a condition was pre-existing.
Laboratory tests
In the literature, at least a hundred different laboratory test parameters have been reported to possibly be influenced by oral contraceptive use, predominantly by the oestrogenic component. Among these are: biochemical parameters of the liver, thyroid, adrenal and renal function, plasma levels of (carrier) proteins and lipid/lipoprotein fractions and parameters of coagulation and fibrinolysis.
In the following conditions the benefit of oral contraception generally outweighs the theoretical or known risk. However, they may need to be considered before prescribing to individual patients:
• Known hyperlipidaemias. A small proportion of women will have persistent hypertriglyceridemia while on the pill. Changes in serum triglycerides and lipoprotein levels have been reported in oral contraceptive users. However, routine screening of women with hypertriglyceridaemia is not considered appropriate.
• Diabetes without vascular involvement (although all patients with diabetes are at increased risk of arterial disease).
• Decreased glucose tolerance. The oestrogen component of Trinovum may cause a decrease in glucose tolerance, while the progestogens may increase insulin secretion and create insulin resistance. Because of these demonstrated effects, pre-diabetic and diabetic women in particular should be carefully monitored while taking oral contraceptives.
• Asymptomatic gall bladder disease or cholecystectomy
• Benign liver tumours (focal nodular hyperplasia). There is limited, direct evidence that hormonal contraceptive use does not influence either progression or regression of liver lesions among women with focal nodular hyperplasia.
• Migraine without focal aura. The onset or exacerbation of migraine or development of headache with a new pattern which is recurrent, persistent or severe requires discontinuation of oral contraceptives and evaluation of the cause.
If any of the following conditions developed or worsened during a prior pregnancy or during previous COC use, they may occur while taking Trinovum:
• elevated blood pressure
• cholestasis
• herpes gestationis
• otosclerosis
• SLE
• severe headaches.
Chloasma
Chloasma may occasionally occur, especially in women with a history of chloasma gravidarum. Women with a tendency to chloasma should avoid exposure to the sun or ultraviolet radiation whilst taking this preparation. Chloasma is often not fully reversible.
Additional contraceptive precautions
When additional contraceptive precautions are required, the patient should be advised either not to have sex, or to use a cap plus spermicide or for her partner to use a condom. Rhythm methods should not be advised as the pill disrupts the usual cyclical
changes associated with the natural menstrual cycle, eg changes in temperature and cervical mucus.
Excipients
The tablets contain lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
4.5. Interaction with other medicinal products and other forms of interaction
Potential Reduction in Contraceptive Effectiveness Associated With CoAdministration of Other Drugs:
Hepatic enzyme inducers
Drugs or herbal products that induce enzymes, especially CYP3A4, may decrease the plasma concentrations of contraceptive hormones, and may decrease their effectiveness and/or increase breakthrough bleeding.
Examples include:
• barbiturates
• bosentan
• carbamazepine
• eslicarbazepine acetate
• felbamate
• (fos)aprepitant
• griseofulvin
• some (combinations of) HIV protease inhibitors (e.g. nelfinavir, ritonavir, ritonvair-boosted protease inhibitors)
• some HCV protease inhibitors (e.g. boceprevir, telaprevir)
• modafinil
• some non-nucleoside reverse transcriptase inhibitors (e.g. nevirapine)
• oxcarbazepine
• phenytoin
• rifampicin and rifabutin
• rufinamide
• St. John’s Wort
• topiramate
Drugs that affect absorption
Colesevelam, a bile acid sequestrant given together with a combined oral hormonal contraceptive, has been shown to significantly decrease the AUC of ethinyl estradiol. No interaction was seen when the contraceptive was given 4 hours before colesevelam.
Management
For women on long-term treatment with drugs and herbal products that interact with hormonal contraception, another reliable, non-hormonal method of contraception is recommended.
Women on short-term treatment with drugs and herbal products that interact with hormonal contraception and may decrease plasma levels of contraceptive hormones could have their contraceptive effectiveness reduced. They should be advised to use a barrier contraceptive method (e.g. condoms, diaphragm) in addition to Trinovum as follows:
• Women using liver enzyme-inducing drugs should temporarily use a barrier contraceptive method in addition to Trinovumthe time of concomitant medicinal product administration and for 28 days after their discontinuation.
• In the case of modafinil, use of a barrier contraceptive method should continue for 56 days after discontinuation.
If discontinuation of the concomitant medicinal product occurs in week three or runs beyond the end of the tablets in the strip, the next strip should be started the next day without a break.
Increase in Plasma Hormone Levels Associated With Co-administered Drugs
• etoricoxib
• some HIV protease inhibitors (e.g. atazanavir, indinavir)
Changes in Plasma Levels of Co-Administered Drugs that may be of Clinical Significance:
Combination hormonal contraceptives may also affect the pharmacokinetics of some other drugs if used concomitantly.
Drugs whose plasma levels may be increased (due to CYP inhibition)
Examples include:
• ciclosporin
• prednisolone
• selegiline
• theophylline
• tizanidine
Drugs whose plasma levels may be decreased (due to induction of glucuronidation) Examples include:
• lamotrigine
Management
Physicians are advised to consult the labelling of concurrently-used drugs to obtain further information about interactions with hormonal contraceptives and the possible need to adjust dosages.
4.6 Pregnancy and lactation
4.6.1 Use during pregnancy
Not indicated during pregnancy. Confirm suspected pregnancy before discontinuing treatment.
The majority of recent studies do not indicate a teratogenic effect, particularly in so far as cardiac anomalies and limb reduction defects are concerned, when taken inadvertently during early pregnancy.
4.6.2 Use during lactation
Contraceptive steroids and/or their metabolites may be excreted in breast milk.
The use of COCs is contraindicated for breast-feeding mothers less than 6 weeks postpartum (see section 4.3) and should be used with clinical judgement for breast-feeding mothers between 6 weeks and 6 months post-partum (see section 4.4).
Mothers who are breast-feeding should be advised not to use the combined pill since this may reduce the amount of breast milk, but may be advised instead to use a progestogen-only pill (POP).
4.7. Effects on Ability to Drive and Use Machines
Not applicable.
4.8 Undesirable effects
The safety of Trinovum Oral Contraceptive Tablets was evaluated in 2542 subjects in 5 clinical trials. Three of the 5 trials were randomised, double-blind, active-controlled trials, 1 was a randomised, double-blind trial comparing 4 different norethisterone/ ethinylestradiol formulations, and 1 was an uncontrolled open-label trial.
Based on safety data from these clinical trials, the most commonly reported Adverse Reactions (ARs) (very common) were headache, vomiting, dysmenorrhea, metrorrhagia, and premenstrual syndrome. the incidence of the ARs of vomiting, dysmenorrhea, metrorrhagia and premenstrual syndrome were highest in cycle 1 and, in general, decreased over time with further treatment cycles (cycle 4 or 6). The event of premenstrual syndrome was not reported after cycle 4.
Including the above-mentioned ARs, the following table displays ARs that have been reported with the use of Trinovum Oral Contraceptive Tablets from either clinical trial or post-marketing experiences.
The displayed frequency categories use the following convention: very common (>1/10), common (>1/100 to <1/10); uncommon (>1/1000 to <1/100); rare (>1/10,000 to <1/1000); very rare (<1/10,000); and not known (cannot be estimated from the available clinical trial data).
|
Infections and infestations | |
|
Uncommon |
Vulvovaginal candidiasisa |
|
Neoplasms benign, malignant and unspecified (including cysts and polyps) | |
|
Uncommon |
Breast mass |
|
Rare |
Cervical dysplasia |
|
Very rare |
Breast cancer, Breast neoplasm, Cervix carcinoma, |
|
Hepatic adenoma, Hepatic neoplasm malignant | |
|
Immune System Disorders | |
|
Uncommon |
Hypersensitivity |
|
Frequency not known |
Anaphylactic / Anaphylactoid reaction |
|
Metabolism and nutrition disorders | |
|
Uncommon |
Fluid retention, Decreased appetite |
|
Rare |
Increased appetite, Inability to lose weight |
|
Frequency not known |
Dyslipidaemia, Glucose tolerance impaired |
|
Psychiatric disorders | |
|
Common |
Depression, Nervousness |
|
Uncommon |
Anxiety, Mood swings, Libido decreased, Mood altered |
|
Nervous system disorders | |
|
Very common |
Headache” |
|
Common |
Dizziness |
|
Uncommon |
Migraine |
|
Frequency not known |
Cerebrovascular accident |
|
Eye disorders | |
|
Rare |
Contact lens intolerance |
|
Frequency not known |
Retinal vascular thrombosis |
|
Cardiac disorders | |
|
Frequency not known |
Myocardial infarction |
|
Vascular disorders | |
|
Common |
Hypertension |
|
Frequency not known |
Deep vein thrombosis |
|
Respiratory, Thoracic, and Mediastinal Disorders | |
|
Frequency not known |
Pulmonary embolism |
|
Gastrointestinal disorders | |
|
Very common |
Vomiting |
|
Common |
Abdominal painc, Abdominal distension, Diarrhoea |
|
Uncommon |
Nausea |
|
Frequency not known |
Pancreatitis, Thrombosis mesenteric vessel |
|
Hepatobiliary disorders | |
|
Rare |
Cholelithiasis, Hepatitis |
|
Frequency not known |
Budd-Chiari syndrome |
|
Skin and subcutaneous tissue disorders | |
|
Common |
Acne |
|
Uncommon |
Alopecia, Chloasma, Pruritus, Rash, Urticaria |
|
Rare |
Hirsutism |
|
Frequency not known |
Angioedema, Erythema nodosum, Photosensitivity reaction, Rash pruritic |
|
Musculoskeletal and Connective Tissue Disorders | |
|
Common |
Back pain, Muscle spasms |
|
Reproductive system and breast disorders | |
|
Very Common |
Dysmenorrhoea, Metrorrhagia, Premenstrual syndrome |
|
Common |
Amenorrhoea, Breast tenderness, Breast pain, Genital discharged Menorrhagia, Uterine cervical erosion, Pelvic pain, Vulvovaginal pruritus Withdrawal bleed |
|
Very rare |
Breast enlargement, Galactorrhoea, Vulvovaginal dryness |
|
Frequency not known |
Menstruation irregular, Menstruation delayed, Suppressed lactation, Vaginal haemorrhage |
|
General disorders and administration site conditions | |
|
Uncommon |
Asthenia, Fatigue, Irritability, Malaise, Oedema, Oedema peripheral |
|
Investigations | |
|
Uncommon |
Weight increased |
|
Rare |
Weight decreased |
a Frequency was determined based on combined number of events for the following AR terms: vulvovaginal candidiasis and candidiasis
b Frequency was determined based on combined number of events for the following AR terms: headache and tension headache
c Frequency was determined based on combined number of events for the following AR terms: abdominal pain and abdominal pain lower
d Frequency was determined based on combined number of events for the following AR terms: genital discharge, cervical discharge and vaginal discharge
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9 Overdose
There have been no reports of serious ill-health from overdose. Symptoms that may occur are nausea, vomiting and vaginal bleeding. There are no antidotes and further treatment should be symptomatic.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Trinovum oral contraceptive tablets act through the mechanism of gonadotrophin suppression by the oestrogenic and progestational actions of the ethinylestradiol and norethisterone.
The primary mechanism of action is inhibition of ovulation, but alterations to the cervical mucus and to the endometrium may also contribute to the efficacy of the product.
5.2 Pharmacokinetic Properties
Norethisterone and ethinylestradiol are absorbed from the gastro-intestinal tract and metabolised in the liver. To obtain maximal contraceptive effectiveness the tablet should be taken as directed and at approximately the same time each day. If the patient has vomiting or diarrhoea, absorption of the hormones will be impaired, making it advisable use an additional reliable method of contraception until her next menstrual Period.
Because the active ingredients are metabolised in the liver, reduced contraceptive efficacy has been associated with concomitant use of oral contraceptives and rifampicin.
A similar association has been suggested with oral contraceptives and barbiturates, phenytoin sodium, phenylbutazone griseofulvin and ampicillin.
5.3. Preclinical Safety Data
No relevant information additional to that contained elsewhere in the summary of characteristics
List of excipients
6.1
White Tablets:
Lactose, Pregelatinised starch, Magnesium stearate, Methanol* Purified water*
Pale Peach Tablets:
Lactose, Pregelatinised starch, FD&C Yellow number 6 (E110), Magnesium stearate, Methanol* Purified water*
Peach Tablets:
Lactose, Pregelatinised starch, FD&C Yellow number 6 (E110), Magnesium stearate, Methanol* Purified water*
(* not detected in final product)
6.2. Incompatibilities
None stated
6.3 Shelf life
2 years
6.4 Special precautions for storage
Do not store above 30°C. Protect from light.
6.5. Nature and Contents of Container
Clear uncoloured PVC/foil Blister strips of 21 tablets. Strips are packaged with a patient information leaflet with or without a plastic or card wallet in a cardboard carton. Park sizes:1x21*,3x21,6x21*,50x21* and 100x21*(* Not marketed)
6.6. Instruction for Use/Handling
None stated
7 MARKETING AUTHORISATION HOLDER
Janssen-Cilag Limited 50-100 Holmers Farm Way High Wycombe Buckinghamshire HP12 4EG UK
8 MARKETING AUTHORISATION NUMBER(S)
PL 00242/0279
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE
AUTHORISATION 01 September 1995
10 DATE OF REVISION OF THE TEXT
15/12/2015