Trintek 15 Glyceryl Trinitrate Transdermal Patch
Package leaflet: Information for the patient
TRINTEK 15 Glyceryl Trinitrate Transdermal Patches Glyceryl trinitrate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What TRINTEK is and what it is used for
2. What you need to know before you use TRINTEK
3. How to use TRINTEK
4. Possible side effects
5. How to store TRINTEK
6. Contents of the pack and other information
The full name of your product is TRINTEK 15 Glyceryl Trinitrate Transdermal Patch, however, throughout the leaflet it will be referred to as TRINTEK.
1. What TRINTEK is and what it is used for
TRINTEK is an adhesive (stick on) patch containing glyceryl trinitrate which is released through the skin and into the body. Glyceryl trinitrate is one of a group of medicines called nitrates which help to widen blood vessels, making blood flow more easily.
TRINTEK patches are used to prevent angina, but are not used to relieve a sudden attack of angina. Angina is a painful tightness in the chest which occurs when the muscles of the heart are not receiving enough blood and oxygen. Pain may also be felt in the neck and arms.
2. What you need to know before you use TRINTEK Do not use TRINTEK if you:
• are allergic to glyceryl trinitrate or other medicines or foods containing nitrates, or any of the other ingredients in the patch (listed in section 6). An allergic reaction may cause a rash, itching or shortness of breath,
• suffer from very low blood pressure or blood volume,
• have obstructive heart disease e.g. narrowing of the valves in your heart (stenosis), inflammation of the heart lining (constrictive pericarditis), thickening of the heart
muscle (hypertrophic cardiomyopathy), build up of fluid or blood in the space between the heart muscle and covering sac of the heart (pericardial tamponade),
• are taking medicines for erectile dysfunction, such as sildenafil, as a severe and possibly dangerous fall in blood pressure could occur,
• are being treated for severe anaemia (have low levels of haemoglobin - a substance contained within the red blood cells which is responsible for carrying oxygen around the body),
• have blood loss or you are in shock,
• have a head injury, raised pressure or bleeding in the brain,
• have an eye condition called closed-angle glaucoma.
Warnings and precautions
Talk to your doctor or pharmacist before using TRINTEK if you have any of the following conditions:
• have had a recent heart attack or heart failure,
• are being treated for lung disease,
• are suffering from any disorder of the thyroid gland, liver or kidney,
• are suffering from malnutrition (severe lack of food),
• hypothermia (very low body temperature),
• sometimes feel dizzy or faint, particularly when getting up from lying or sitting (postural hypotension).
If you are having certain procedures which use magnetic or electrical fields like a magnetic resonance imaging scan (MRI scan), cardioversion or defibrillation (an electric shock used to return the heart to its normal rhythm) or diathermy (where body tissues are heated electrically to treat or help certain conditions), you will need to remove your patch.
Children and adolescents
TRINTEK patches are not recommended for use in children.
Other medicines and TRINTEK
Some medicines can affect the way TRINTEK works, or TRINTEK can affect other medicines taken at the same time.
Do not take medicines for erectile dysfunction such as sildenafil while you are using TRINTEK, as a severe and possibly dangerous fall in blood pressure could occur. This could result in collapse, unconsciousness or even death.
Tell your doctor if you are taking:
• medicines for lowering blood pressure, water retention or for heart problems like calcium channel blockers, ACE inhibitors, beta-blockers, and diuretics,
• medicines for anxiety or depression (e.g. tricyclic antidepressants, tranquillisers),
• amifostine (a medicine used to reduce the side effects of certain cancer treatments or radiotherapy),
• medicines for migraine containing ergotamine. Taking TRINTEK with dihydroergotamine, a medicine used to treat migraine, can affect the blood vessels in the heart.
• aspirin,
• non-steroidal anti-inflammatory drugs, NSAIDs (used to treat pain and inflammation)
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
TRINTEK with food, drink and alcohol
Drinking alcohol when using TRINTEK is not recommended, as this can increase the fall in blood pressure that may occur when using the patch. TRINTEK is not affected by food.
Pregnancy and breast-feeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
There is limited information on the excretion of this medicine in breast milk.
Driving and using machines
TRINTEK may sometimes impair your reactions or cause dizziness, light-headedness upon standing or fainting or blurred vision, especially at the start of treatment or when the dose is adjusted. If you experience these symptoms, do not drive or use any tools or machinery.
3. How to use TRINTEK™
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
• This patch is used for preventing angina and not for treating a sudden attack. Your doctor will have prescribed another medicine to treat sudden attacks of angina.
• TRINTEK patches are for use in adults only and are not recommended for use in children.
• The recommended starting dose is one TRINTEK 5 mg transdermal patch per day. Your doctor may increase the dose gradually if necessary.
• If higher doses are required to prevent your angina, a TRINTEK 10 mg transdermal patch or a TRINTEK 15 mg transdermal patch may be prescribed. The maximum dose is one TRINTEK 15 mg transdermal patch per day. Never use more patches or wear them for longer than your doctor has advised. You should check with your doctor if you are not sure.
• You should apply the patch at the same time each morning. The patch should normally be worn for up to 16 hours during the day and removed when you go to bed. This means that you have a patch-free time of at least 8 hours in each 24 hour period.
• If you are also taking other medicines for your angina your doctor will advise you on the best time to take them.
• Select a clean, dry, relatively hairless area on either side of your chest. If hair is likely to interfere with patch adhesion or removal, it can be clipped but not shaved.
• Take care to avoid areas with cuts or irritations.
• Do not apply the patch immediately after showering or bathing. It is best to wait until you are certain the skin is completely dry.
Do not tear or cut your patch.
How to apply the patch
1. Each TRINTEK patch is individually sealed in a protective package.
• Open the pouch at the tear mark.
• Carefully remove the patch.
• The patch is printed with the wording TRINTEK 15, the patch size (24cm2) and the amount delivered in 24 hours (15mg in 24hr).
• The patch is attached to a clear peelable liner.
• The liner has a slit which divides it into two strips.
• Hold the patch with the wording facing away from you.
• The slit should now be facing toward you.
• Rotate the patch as necessary to place the slit in an up and down position.
TRINTEK 15 15mg/24hr (24cm2)
V_)
2. Bend both sides of the clear peelable liner away from you at the slit.
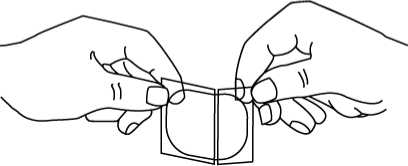
3. Slowly peel off only one of the strips of the clear liner. Do not touch the exposed side of the patch.
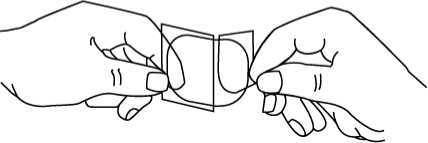
4. Using the remaining strip as a handle , apply the sticky side of the patch to the skin. Press the sticky side on the chosen skin site and smooth down.
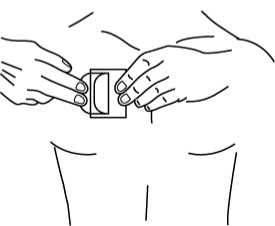
5.
• Fold back the unattached side of the patch.
• Grasp the remaining strip and remove it while applying the remainder of the patch to the skin.
• Press the patch on the skin and smooth down.
• Once the patch is in place, do not test how well it is stuck on by pulling on it.
• When the TRINTEK patch is applied to your body, the active ingredient contained in the patch begins to flow from the sticky surface through your skin at a regular rate.
6. After applying the patch, wash your hands to remove any drug.
Please Note: Once the patch is in place, swimming, showering or bathing should not affect it. However, if the patch does fall off, dispose of it carefully and put a new patch on a different skin site.
Changing your patch
• Remove your patch at the time recommended by your doctor. Dispose of the used patch carefully as it will still contain some of the active ingredient. Fold the patch in half with sticky sides together before disposing it.
• Place a new patch on a different skin site (following steps 1 through to 6 above) at the time recommended by your doctor.
• Try not to apply the new patch to the same area of the skin used for the previous patch and leave one week before putting a patch in an area of the skin already used.
If you forget to use your patch
If you forget to apply your patch in the morning you may do so later in the day. If you have missed a day do not apply extra patches to make up for the day missed.
If you accidentally use too many patches
If you accidentally use too many patches remove the extra patches, then tell your doctor immediately or go to the nearest hospital casualty department, even if you do not feel unwell. Always take the labelled medicine package with you, whether there is any TRINTEK left or not.
Too much glyceryl trinitrate can cause you to feel sick, be sick and feel restless. Other symptoms include warm, flushed skin, blurred vision, headache, confusion, low blood pressure with a fast or irregular heart rate which may make you feel light-headed or cause you to faint or collapse.
If you stop using the patch
Do not stop using the patches suddenly as this could cause an angina attack or a heart attack. Always consult your doctor first.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine may cause side effects, although not everybody gets them.
All medicines including TRINTEK can cause allergic reactions. You should contact your doctor immediately if you experience any of the following symptoms after taking TRINTEK:
• sudden wheeziness
• difficulty in breathing or dizziness
• swelling of the eyelids, face, lips or throat.
Other side effects:
Very common (may affect more than 1 in 10 people):
• Nausea (feeling sick).
• Vomiting (being sick).
Common (may affect up to 1 in 10 people):
• Headache, especially at the start of treatment. This usually wears off after a few days but you can take some mild pain killers (such as paracetamol) for this. If the headaches continue or are very severe, consult your doctor.
Uncommon (may affect up to 1 in 100 people):
• Skin irritation, a burning sensation, itchy rash or reddening where you have placed the patch. It is therefore important that you choose a different place on your skin to put the patch every day.
• Inflammation of the skin with symptoms such as red, itchy, scaly skin (contact dermatitis).
Rare (may affect up to 1 in 1,000 people):
• Flushing .
• Rapid heart beat or pulse (tachycardia). Sometimes your heart beat may slow and your angina may temporarily get worse.
• Faintness, dizziness or light-headedness may occur, especially when suddenly rising from the lying position (orthostatic or postural hypotension).
Very rare (may affect up to 1 in 10,000 people):
• Dizziness.
Not known (frequency cannot be estimated from available data)
• Fast or irregular heart beats called palpitations.
• Rash.
Reporting of side effects:
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the internet at www.mhra.gov.uk/yellowcard. Alternatively you can call Freephone 0808 100 3352 (available from 10 a.m. to 2 p.m. Mondays to Fridays) or fill in a paper form available from your local pharmacy.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store TRINTEK
• Keep this medicine out of the sight and reach of children.
• Do not use this medicine after the expiry date, which is stated on the package. The expiry date refers to the last day of that month.
• Do not store above 25°C. Do not refrigerate.
• Do not store patches once they have been removed from the protective pouch.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
Contents of the pack and other information
6.
What TRINTEKcontains
Each TRINTEKtransdermal patch contains glyceryl trinitrate as the active ingredient. The amount of active ingredient varies according to the size of the patch:
TRINTEK 15 (24cm2 transdermal patch) contains 67.2mg which releases 15mg of active ingredient over 24 hours.
The inactive ingredients in the patch are:
Release liner consisting of a polyester layer coated on one side with silicone. Backing film made from polyethylene resins. Adhesive made from a 2-ethylhexylacrylate, vinyl acetate and acrylic acid.
White ink containing: titanium dioxide (pigment), acrylic polymer resin, styrenated acrylic polymer emulsion, triethanolamine, propylene glycol, polyethylene wax, polytetrafluorethylene, polydimethylsiloxane and sodium dioctyl sulphosuccinate.
What TRINTEK looks like and contents of the pack
TRINTEK 15 is a rectangular patch with rounded corners. Each patch is marked with “TRINTEK15 15mg/24hr (24cm2)” in white ink. The patches are individually wrapped in a foil-lined pouch and are supplied in cardboard cartons containing 15 or 30 patches.
Marketing Authorisation Holder
Generics [UK] Limited t/a Mylan, Potters Bar, Hertfordshire, EN6 1TL, United Kingdom Manufacturer
McDermott Laboratories Ltd t/a Gerard Laboratories, 35/36 Baldoyle Industrial Estate, Grange
Road, Dublin 13, Ireland
and
Generics [UK] Limited, Station Close, Potters Bar, Hertfordshire, EN6 1TL, United Kingdom This leaflet was last revised in: June 2013
7