Trisequens Tablets
Out of date information, search anotherPatient Information Leaflet Trisequens® Tablets
(estradiol hemihydrate and norethisterone acetate)
Read all of this leaflet carefully before you start taking this medicine.
• Keep this leaflet. You may need to read it again
• If you have further questions, ask your doctor or pharmacist
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Trisequens is and what it is used for
2. Before you take Trisequens
3. How to take Trisequens
4. Possible side effects
5. Howto store Trisequens
6. Further information
1. What Trisequens is and what it is used for
Trisequens is a sequential combined Hormone Replacement Therapy (HRT), which is taken every day without interruption. Trisequens is suitable for postmenopausal women with at least 6 months since their last natural period.
Trisequens contains the female sex hormone estradiol and norethisterone acetate. The estradiol in Trisequens is identical to the estradiol in the ovaries of women, and is classified as a natural oestrogen. Norethisterone acetate is a synthetic progestogen, which acts in a similar manner as the body’s own hormone progesterone, another important female sex hormone.
Trisequens is indicated:
• To relieve unpleasant symptoms of menopause like hot flushes, night sweats, vaginal dryness in postmenopausal women who still have their womb.
• For the prevention of osteoporosis (thinning of the bones) in postmenopausal women if they are at high risk of future fractures and if they are unable to take other medications for this purpose.
The experience of treating women older than 65 years is limited.
2. Before you take Trisequens Safety of HRT
As well as benefits, HRT has some risks which you need to consider when you’re deciding whether to take it, or whether to carry on taking it.
Medical check-ups
Before you start taking HRT, your doctor should ask about your own and your family’s medical history. Your doctor may decide to examine your breasts and/or your abdomen, and may do an internal examination - but only if these examinations are necessary for you, or if you have any special concerns.
Once you have started on HRT, you should see your doctor for regular check-ups (at least once a year). At these check-ups, your doctor may discuss with you the benefits and risks of continuing with Trisequens.
As well as regular check-ups with your doctor, be sure to:
• Regularly check your breasts for any changes such as dimpling of the skin, changes in the nipple, or any lumps you can see or feel.
• Go for regular breast screening (mammography) and cervical smear tests.
Do not take Trisequens
If any of the following applies to you, talk to your doctor. Do not start taking Trisequens:
• If you have, have had or suspect having breast cancer,
• If you have, have had, or suspect having cancer of the womb lining (endometrial cancer), or any other oestrogen dependent cancer.
• If you have abnormal vaginal bleeding of an unknown cause.
• If you have endometrial hyperplasia (excessive growth of the womb lining) that is not being treated
• If you have or have had blood clots in a vein (venous thromboembolism), in the legs (deep venous thrombosis) or the lungs (pulmonary embolism).
• If you have a blood clotting disorder (thrombophilic disorder, such as protein C, protein S, or antithrombin deficiency).
• If you have or have had a heart attack, stroke, or have angina pectoris which causes discomfort, pressure or pain in the chest.
• If you have or have had liver problems and your liver function tests have not returned to normal
• If you have porphyria (a liver enzyme disease)
• If you are allergic (hypersensitive) to estradiol, norethisterone acetate or any other ingredients in Trisequens (listed in section 6,“Further information’’).
Take special care with Trisequens
If you have (or have had) any of the following conditions, tell your doctor. Your doctor may want to monitor you more closely. These conditions may in rare cases come back or get worse during treatment with Trisequens:
• If you have or have had leiomyoma (benign tumours of the womb) or endometriosis, a condition in which the lining of the uterus (womb) grows outside the uterus, causing pain or bleeding.
• If you have a history of blood clots (thrombosis) or have risk factors for blood clots (see blood clots in a vein) (these risk factors and symptoms for a blood clot are listed in section 4," other side effects of combined HRT”)
• If you have risk factors for development of oestrogen dependent tumours, such as immediate relatives (mother, sister, maternal or paternal grandmother) with breast and/or endometrial cancer.
• If you have high blood pressure
• If you have a liver disorder such as liver adenoma (a benign tumour)
• If you have diabetes mellitus with or without vascular disorders
• If you have gallstones
• If you have migraines or severe headache
• If you have systemic lupus erythematosus (SLE) - an
autoimmune disease.
• If you previously have had endometrial hyperplasia
(excessive growth of the lining of the womb).
• If you have epilepsy.
• If you have asthma
• If you have otosclerosis (progressive hearing loss).
If you need a blood test, tell your doctor that you are taking Trisequens, since oestrogen can affect the results of certain laboratory tests.
If you are going to have surgery, talk to your doctor. You may need to stop taking these tablets 4 to 6 weeks before the operation, to reduce the risk of a blood clot. Your doctor will tell you when you can start HRT again.
HRT and cancer
Excessive growth of the lining of the womb (endometrial hyperplasia) and cancer of the womb lining (endometrial cancer)
In women with an intact womb taking oestrogen-only HRT over a long period of time, the risk of excessive growth of the lining of the womb (endometrial hyperplasia) and cancer of the womb lining (endometrial cancer) is increased.
Taking a progestogen in addition to the oestrogen, such as Trisequens, helps to lower the extra risk.
Compare
In women who still have a womb and who are not taking HRT, on average 5 in 1,000 will be diagnosed with endometrial cancer.
For women who still have a womb and take oestrogen-only HRT, the number of extra cases could vary between 5 and 55 in 1,000 users between the ages of 50 and 65 depending on the dose and for how long it is taken.
The addition of a progestogen to oestrogen-only HRT substantially reduces the risk of endometrial cancer.
Breast cancer
Evidence suggests that taking combined oestrogen-progestogen and possibly also oestrogen-only HRT increases the risk of breast cancer.
This depends on how long you take HRT. The additional risk is visible after about 3 years. However, it returns to normal within a few years (at most 5) after stopping treatment.
Compare
Women aged 50 to 65 who are not taking HRT, on average 9 to 12 in 1,000 will be diagnosed with breast cancer over a 5-year period.
For women aged 50 to 65 who are taking oestrogen-progestogen HRT over 5 years, the number of extra cases will be 6 in 1,000 users.
Looking at women aged 50 to 79 who are not taking HRT, on average 14 in 1,000 will be diagnosed with breast cancer over a 5-year period.
For women aged 50 to 79 who are taking oestrogen-progestogen HRT over 5 years, the number of extra cases will be 4 in 1,000 users.
Ovarian cancer
Ovarian cancer is much rarer than breast cancer. Long-term (at least 5 to 10 years) use of oestrogen-only HRT products is thought to carry a slightly increased risk of ovarian cancer. Some studies suggest that the long-term use of combined HRT may carry a similar or slightly smaller risk.
For women who have been taking HRT for over 5 years there will be 1 extra case per 2,500 users.
Effect of HRT on heart and circulation
Blood clots in a vein (venous thromboembolism)
HRT increases the risk of blood clots in the veins 1.3- to 3-fold, especially during the first year of taking it.
You are generally more likely to get a blood clot in your veins if one or more of the following applies to you:
• You are older
• You are pregnant or have recently had a baby
• You use oestrogens
• You or any of your close relatives have ever had a blood clot in the leg, lung or another organ
• You are seriously overweight
• You have systemic lupus erythematosus (SLE)
• You have a blood clotting problem that needs long-term treatment with a medicine used to prevent blood clots (anticoagulant)
• You are unable to walk or stand for a long time because of major surgery, injury or illness (prolonged immobilisation)
• You have cancer
Compare
Women in their 50s who are not taking HRT, on average over a 5-year period, 4 in 1,000 would be expected to get a blood clot in a vein.
For women in their 50s who have been taking oestrogen-progestogen HRT for over 5 years, the number of extra cases will be 5 in 1,000 users.
Coronary artery disease (CAD)
There is no evidence that HRT will help to prevent heart disease. Women taking oestrogen-progestogen HRT are slightly more likely to develop heart disease than those not taking any HRT. As the baseline absolute risk of heart disease strongly depends on age, the number of extra cases of heart disease due to use of oestrogen-progestogen HRT is very low in healthy women close to menopause, but will rise with more advanced age.
Stroke
Combined oestrogen-progestogen and oestrogen-only HRT increase the risk of stroke up to 1.5-fold. The comparable risk for users versus non-users does not change with age or time since menopause. However, as the risk of stroke is strongly age related, the overall general risk of stroke in women who use HRT will increase with age.
Compare
Women in their 50s who are not taking HRT, on average 8 in 1,000 would be expected to have a stroke over a 5-year period. For women in their 50s who are taking HRT, the number of extra cases will be 3 in 1,000 users over 5 years.
Other conditions
HRT will not prevent memory loss.
The risk of probable memory loss may be somewhat higher in women who start using any kind of HRT after the age of 65.
Stop taking Trisequens
If you experience any of the following conditions below, stop taking Trisequens, and contact your doctor immediately.
• If you get a migraine-type headache for the first time.
• If you develop yellow skin or eyes (jaundice) or other liver problems.
• If your blood pressure goes up significantly while you are taking Trisequens (symptoms of high blood pressure e.g. headache, tiredness and dizziness).
• If you become pregnant.
• If you experience any of the conditions listed in Section 2 Before you take Trisequens
Bleeding with Trisequens
Trisequens will cause a menstruation-like monthly bleeding, which usually occurs at the beginning of a new pack. If the periods get heavier than normal, you should tell your doctor. However, some women may also experience breakthrough bleeding or spotting during the first few months of taking Trisequens. This kind of bleeding is different from the menstruation-like bleeding. If you have any breakthrough bleeding or spotting that continues for longer than the first few months, starts after some time on HRT, or continues even if you have stopped taking Trisequens, you should tell your doctor as soon as possible.
Using other medicines
Some medicines may reduce the effects of Trisequens:
• Drugs used for epilepsy (such as phenobarbital, phenytoin and carbamazepine)
• Drugs used for tuberculosis (such as rifampicin, rifabutin)
• Drugs used for HIV infections (such as nevirapine, efavirenz, ritonavir and nelfinavir)
• Herbal products with St John’s Wort (Hypericum perforatum).
Other medicines may increase the effects of Trisequens:
• Drugs containing ketoconazole (a fungicide).
Concomitant administration of ciclosporine may cause increased blood levels of cyclosporine.
Please tell your doctor or pharmacist, if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Taking Trisequens with food and drink
The tablets can be taken with or without food and drink,
Pregnancy and breast-feeding
Pregnancy: You should not take Trisequens if you are pregnant. If you become pregnant while using Trisequens, you should stop the treatment immediately and contact your doctor. Breast-feeding: You should not use Trisequens if you are breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
Trisequens does not affect the use of any machines or the ability to drive safely.
Important information about some of the ingredients of Trisequens:
Trisequens contains lactose monohydrate. If you have an intolerance to some sugars, contact your doctor before taking Trisequens.
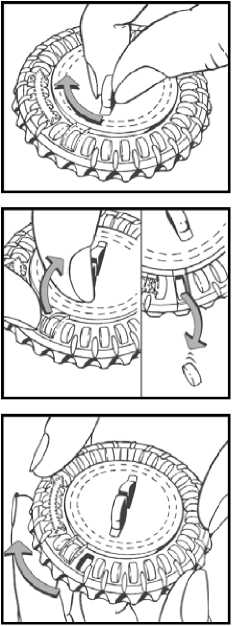
1. Set the day reminder
Turn the inner disc to set the day of the week opposite the little plastic tab.
3. How to use Trisequens
Always take Trisequens exactly as your doctor has told you. Check with your doctor or pharmacist if you are unsure.
Take one tablet once a day, at about the same time each day
Take the tablet with a glass of water.
Take a tablet every day without stopping. Begin by taking the blue tablets for 12 days followed by the white tablets for 10 days and finally the red tablets for 6 days.
After taking the last red tablet, treatment is continued with the first blue tablet of a new calendar pack on the next day without interruption.
You should start treatment with Trisequens on the fifth day of your bleed (whether it has stopped or not) if you are still having periods, or if you are switching from an HRT product where you have a monthly bleed. If you no longer have periods or they are infrequent, you can start treatment with Trisequens on any convenient day. This also applies if you are switching from an HRT product where you do not have a monthly bleed.
Your doctor should aim to prescribe the lowest dose for the shortest time that gives you relief from your symptoms.
Talk to your doctor if your symptoms are not better after three months.
The tablet comes in calendar dial pack. A translation of the days of the week is as follows:
LU MA ME JE VE SA Dl
MON TUE WED THUR FRI SAT SUN
USER INSTRUCTIONS How to use the calendar pack
2. Take the first day’s tablet
Break the plastic and tip out the first tablet (blue)
3. Move the dial every day
On the next day simply move the transparent dial clockwise 1 space as indicated by the arrow. Tip out the next tablet. Remember to take only 1 tablet once a day.
You can turn the transparent dial after the tablet in the opening has been removed.
If you take more Trisequens than you should
If you have taken more Trisequens than you should, talk to a doctor or pharmacist. An overdose of Trisequens could make you feel sick or vomit.
If you forget to take Trisequens
Do not take double dose to make up for a forgotten individual dose, if you forget to take your tablet at the usual time, take it within the next 12 hours. If more than 12 hours have gone by, start again as normal the next day. Do not take a double dose to make up for a forgotten tablet. Forgetting a dose may increase the likelihood of breakthrough bleeding and spotting.
If you stop taking Trisequens
If you would like to stop your treatment with Trisequens for any reason, please discuss your decision with your doctor, who will explain the effects of stopping treatment and discuss other possibilities with you. If you have any further questions on the use of this product ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Trisequens can have side effects, although not everybody gets them.
Hypersensitivity/allergy
(uncommon side effects - Affects 1 to 10 users in 1,000)
Though it is an uncommon event, hypersensitivity/allergy may occur.
Sign of hypersensitivity/allergy may include one or more of the following symptoms: hives, itching, swelling, difficulty in breathing, low blood pressure (paleness and coldness of skin, rapid heart beat), feeling dizzy, sweating, which could be signs of anaphylactic reaction/shock. If one of the mentioned symptoms appears, stop taking Trisequens and seek immediate medical help.
The frequency of possible side effects listed below is defined using the following convention:
Very common (affects more than 1 user in 10)
Common (affects 1 to 10 users in 100)
Uncommon (affects 1 to 10 users in 1,000)
Rare (affects 1 to 10 users in 10,000)
Very rare (affects less than 1 user in 10,000)
Not known (frequency cannot be estimated from the available data)
Very common side effects
• Breast pain or breast tenderness
• Irregular menstrual periods or excessive bleeding during your periods.
Common side effects
• Headache
• Weight gain caused by fluid retention
• Vaginal inflammation (thrush)
• Vaginal infection with a fungus
• Migraine, new or worse than before
• Depression, new or worse than before
• Nausea
• Abdominal pain, swelling or discomfort
• Enlargement or swelling of the breasts (breast oedema)
• Back pain
• Leg cramps
• Uterine fibroids (benign tumour of the womb), aggravation, occurrence or reoccurrence
• Swelling of arms or legs (peripheral oedema)
• Weight increase
Uncommon side effects
• Bloating or flatulence
• Acne
• Hair loss (alopecia)
• Abnormal (male pattern) hair growth
• Itching or hives (urticaria)
• Inflammation of a vein (superficial thrombophlebitis)
• Drug ineffective
• Allergic reaction
• Painful periods
• Endometrial hyperplasia (excessive growth of the lining of the
womb)
• Nervousness Rare side effects
• Pulmonary embolism (blood clot) (See also Blood clots in section 2 Before you take Trisequens)
• Deep inflammation of a vein associated with thrombosis (blood clot)
POM
Very rare side effects
• Cancer of the lining of the womb (endometrial cancer).
• Increase in blood pressure or worsening of high blood pressure
• Gallbladder disease, gallstones occurrence/reoccurrence or aggravated
• Excessive secretion of sebum, skin eruption
• Acute or recurring attack of oedema (angioneurotic oedema)
• Insomnia, dizziness, anxiety
• Changes in sexual desire
• Visual disturbances
• Weight decreased
• Vomiting
• Heartburn
• Vaginal and genital itching
• Heart attack and stroke
Other side effects of combined HRT
Women using HRT have a slightly increased risk of developing the following diseases:
• Breast cancer (see also section 2 HRT and cancer, Breast cancer for more information).
• Excessive growth or cancer of the lining of the womb (endometrial hyperplasia or cancer) (see also section 2 HRT and cancer, Excessive growth of the lining of the womb (endometrial hyperplasia) and cancer of the womb lining (endometrial cancer) for more information).
• Ovarian cancer, (see also section 2 HRT and cancer, Ovarian cancer for more information).
• Blood clots in the veins of the legs or lungs (venous thromboembolism) (see also section 2 Effects of HRT on heart and circulation, Blood clots in a vein (venous thromboembolism) for more information)
• Heart disease (see also section 2 Effects of HRT on heart and circulation, Coronary artery disease (CAD) for more information)
• Stroke (see also section 2 Effects of HRT on heart and circulation, Stroke for more information).
• Skin and subcutaneous disorders:
- Darkening of the skin (chloasma)
- Severe condition of the skin that may affect the mouth and other parts of the body (erythema multiforme)
- Red-purple swellings on the shins, thighs and, less commonly, the arms. Joint and muscle pains and fever may also occur (erythema nodosum)
- Purple or red-brown spots visible through the skin (vascular purpura)
• Probable memory loss if HRT is started over the age of 65
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. How to store your Trisequens
Do not store above 25°C. Protect from light and moisture. Do not refrigerate.
Keep out of the reach and sight of children
Do not remove the tablets from the calendar dial pack except when taking them.
Do not use after the expiry date on the carton, or if the product shows signs of deterioration or discolouration.
If your doctor decides to stop treatment return any remaining tablets you have left to the pharmacist for safe disposal. Only keep them if your doctor tells you to.
Medicines should not be disposed of via waste water or household waste. Ask your pharmacist howto dispose of medicines no longer required. These measures will help to protect the environment.
6. Further Information
Trisequens 12 blue round film coated tablets marked ‘NOVO 280’ on one side and plain on reverse, each containing 2 milligrams of estradiol (as hemihydrate) which is an oestrogen as active ingredient.
Trisequens 10 white round film coated tablets marked ' NOVO 28T on one side and plain on reverse, each containing 2 milligrams of estradiol (as hemihydrate) and 1mg of norethisterone acetate as active ingredients.
Trisequens 6 red film coated tablets marked ‘NOVO 282’ on one side and plain on reverse, each containing 1 milligram of estradiol (as hemihydrate) as active ingredient.
Each tablet also contains lactose monohydrate, maize starch, hydroxypropyl cellulose, talc, hypromellose and magnesium stearate.
The blue tablets also contain: titanium dioxide (E171), indigo carmine (E132) and macrogol 400 The white tablets also contain: triacetin
The red pills also contain: titanium dioxide (E171), red iron oxide and propylene glycol
Trisequens Tablets come in 3 calendar dial packs of 28 tablets (a total of 84 Tablets).
This medicine is manufactured by Novo Nordisk A/S, 2880 Bagsvaerd, Denmark. Procured from within the EU: Product Licence holder: Quadrant Pharmaceuticals Ltd, Lynstock House, LynstockWay, Lostock, Bolton, BL6 4SA. Repackaged by Maxearn Ltd, Bolton BL6 4SA.
Trisequens Tablets PL 20774/0640
Date of preparation: 20th May 2015
Trisequens is a registered trade mark of Novo Nordisk FemCare AG.
PP3/0640/V2