Trusopt 20 Mg/Ml Eye Drops Solution


Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What TRUSOPT is and what it is used for
2. What you need to know before you use TRUSOPT
3. How to use TRUSOPT
4. Possible side effects
5. How to store TRUSOPT
6. Contents of the pack and other information
TRUSOPT contains dorzolamide which belongs to a group of medicines called "carbonic anhydrase inhibitors”.
This medicine is prescribed to lower raised pressure in the eye and to treat glaucoma. This medicine can be used alone or in addition to other medicines which lower the pressure in the eye (so-called beta-blockers).
Do not use TRUSOPT:
- if you are allergic to dorzolamide hydrochloride or any of the other ingredients of this medicine (listed in section 6).
- if you have severe kidney impairment or problems, or a prior history of kidney stones.
Warnings and precautions
Talk to your doctor or pharmacist before using TRUSOPT.
Tell your doctor or pharmacist about any medical problems you have now or have had in the past, including eye problems and eye surgeries, and about any allergies to any medications.
If you develop any eye irritation or any new eye problems such as redness of the eye or swelling of the eyelids, contact your doctor immediately.
If you suspect that TRUSOPT is causing an allergic reaction (for example, skin rash, severe skin reaction or itching), stop using this medicine and contact your doctor immediately.
Use in children
TRUSOPT has been studied in infants and children less than 6 years of age who have raised pressure in the eye(s) or have been diagnosed with glaucoma. For more information, talk to your doctor.
Use in elderly
In studies with TRUSOPT, the effects of this medicine were similar in both elderly and younger patients.
Use in patients with liver impairment
Tell your doctor about any liver problems you now have or have suffered from in the past.
Other medicines and TRUSOPT
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines (including eye drops). This is particularly important if you are taking another carbonic anhydrase inhibitor such as acetazolamide, or a sulpha drug.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
Use in pregnancy
You should not use this medicine during pregnancy. Tell your doctor if you are pregnant or intend to become pregnant.
Use in breast-feeding
If treatment with this medicine is required, breast-feeding is not recommended. Tell your doctor if you are breast-feeding or intend to breast-feed.
Driving and using machines
No studies on the effects on the ability to drive or use machines have been performed. There are side effects associated with TRUSOPT, such as dizziness and blurred vision, which may affect your ability to drive and/or operate machinery. Do not drive or operate machinery until you feel well or your vision is clear.
TRUSOPT contains benzalkonium chloride
TRUSOPT contains the preservative benzalkonium chloride. This preservative may be deposited in soft contact lenses and may possibly discolour the lenses. If you wear contact lenses, you should consult your doctor before using this medicine.
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure. The appropriate dosage and duration of treatment will be established by your doctor.
When this medicine is used alone, the recommended dose is one drop in the affected eye(s) in the morning, in the afternoon and in the evening.
If your doctor has recommended you use this medicine with a beta-blocker eye drop to lower eye pressure, then the recommended dose is one drop of TRUSOPT in the affected eye(s) in the morning and in the evening.
If you are using TRUSOPT with another eye drop, the drops should be instilled at least 10 minutes apart.
Do not allow the tip of the container to touch the eye or areas around the eye. It may become contaminated with bacteria that can cause eye infections leading to serious damage of the eye, even loss of vision. To avoid possible contamination, wash your hands before using this medicine and keep the tip of the container away from contact with any surface. If you think your medication may be contaminated, or if you develop an eye infection, contact your doctor immediately concerning continued use of this bottle.
Instructions for use:
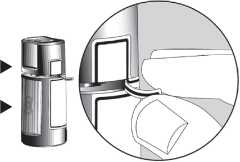
1. Before using the medication for the first time, be sure the Safety Strip on the front of the bottle is unbroken. A gap between the bottle and the cap is normal for an unopened bottle.
Opening Arrows ► Safety Strip ►

2. First wash your hands, then tear off the Safety Strip to break the seal.
Gap
Finger Push Area
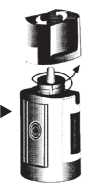
3. To open the bottle, unscrew the cap by turning as indicated by the arrows on the top of the cap. Do not pull the cap directly up and away from the bottle. Pulling the cap directly up will prevent your dispenser from operating properly.
Finger Push Area
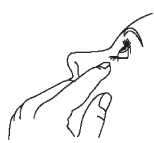
4. Tilt your head back and pull your lower eyelid down slightly to form a pocket between your eyelid and your eye.
5. Invert the bottle, and press lightly with the thumb or index finger over the "Finger Push Area" (as shown) until a single drop is dispensed into the eye as directed by your doctor.
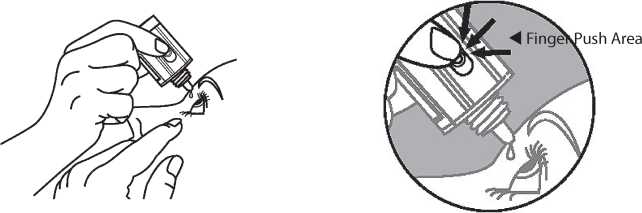
DO NOT TOUCH YOUR EYE OR EYELID WITH THE DROPPER TIP.
6. If drop dispensing is difficult after opening for the first time, replace the cap on the bottle and tighten (Do not overtighten) and then remove by turning the cap in the opposite directions as indicated by the arrows on the top of the cap.
7. Repeat steps 4 & 5 with the other eye if instructed to do so by your doctor.
8. Replace the cap by turning until it is firmly touching the bottle. The arrow on the left side of the cap must be aligned with the arrow on the left side of the bottle label for proper closure. Do not overtighten or you may damage the bottle and cap.
9. The dispenser tip is designed to provide a single drop; therefore, do NOT enlarge the hole of the dispenser tip.
10. After you have used all doses, there will be some medicine left in the bottle. You should not be concerned since an extra amount of medicine has been added and you will get the full amount of medicine that your doctor prescribed. Do not attempt to remove the excess medicine from the bottle.
If you use more TRUSOPT than you should
If you put too many drops in your eye or swallow any of the contents of the container, you should contact your
doctor immediately.
If you forget to use TRUSOPT
It is important to take this medicine as prescribed by your doctor. If you miss a dose, take it as soon as possible.
However, if it is almost time for the next dose, skip the missed dose and go back to your regular dosing
schedule.
Do not take a double dose to make up for the forgotten dose.
If you stop using TRUSOPT
If you want to stop using this medicine talk to your doctor first. If you have any further questions on the use of
this product, ask your doctor or pharmacist.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you develop allergic reactions including hives, swelling of the face, lips, tongue, and/or throat which may cause difficulty in breathing or swallowing, you should stop using this medicine and seek immediate medical advice.
The following side effects have been reported with TRUSOPT either during clinical trials or during postmarketing experience:
Very Common side effects: (affects more than 1 user in 10)
Burning and stinging of the eyes.
Common side effects: (affects 1 to 10 users in 100)
Disease of the cornea with sore eye and blurred vision (superficial punctuate keratitis), discharge with itching of the eyes (conjunctivitis), irritation/inflammation of the eyelid, blurred vision, headache, nausea, bitter taste, and fatigue.
Uncommon side effects: (affects 1 to 10 users in 1,000)
Inflammation of the iris.
Rare side effects: (affects 1 to 10 user in 10,000)
Tingling or numbness of the hands or feet, temporary shortsightedness which may resolve when treatment is stopped, development of fluid under the retina (choroidal detachment, following filtration surgery), eye pain, eyelid crusting, low pressure in the eye, swelling of the cornea (with symptoms of visual disturbances), eye irritation including redness, kidney stones, dizziness, nose bleed, throat irritation, dry mouth, localized skin rash (contact dermatitis), severe skin reactions, allergic type reactions such as rash, hives, itching, in rare cases possible swelling of the lips, eyes and mouth, shortness of breath, and more rarely wheezing.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly (see details below). By reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom: Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
Ireland: HPRA Pharmacovigilence, Earlsfort Terrace, IRL - Dublin 2, Tel: +353 1 6764971, Fax: +353 1 6762517, Website: www.hpra.ie, e-mail: medsafety@hpra.ie
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated by the six numbers following EX (or EXP) on the bottle and outer carton. The first two numbers indicate the month; the last four numbers indicate the year. The expiry date refers to the last day of the month.
TRUSOPT should be used within 28 days after the bottle is opened.
This medicinal product does not require any special temperature storage conditions.
Store bottle in outer carton in order to protect from light.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
What TRUSOPT contains
- The active substance is dorzolamide.
- Each ml contains 22.26 mg of dorzolamide hydrochloride equivalent to 20 mg of dorzolamide.
- The other ingredients are hydroxyethyl cellulose, mannitol, sodium citrate, sodium hydroxide, and water for injections. Benzalkonium chloride is added as a preservative.
What TRUSOPT looks like and contents of the pack
TRUSOPT is a clear, colourless to nearly colourless slightly viscous solution. The OCUMETER Plus Ophthalmic Dispenser consists of a translucent high-density polyethylene container containing 5 ml of solution. Tamper evidence is provided by a safety strip on the container label.
Pack sizes:
1 x 5.0 ml (single 5 ml container)
3 x 5.0 ml (three 5 ml containers)
6 x 5.0 ml (six 5 ml containers)
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder in UK and Ireland: Santen Oy, Niittyhaankatu 20, 33720 Tampere, Finland
Manufacturer: Merck Manufacturing Division, Laboratoires Merck Sharp & Dohme-Chibret, Mirabel Plant, Route de Marsat, Riom 63963 Clermont Ferrand Cedex 9, France.
This medicinal product is authorised in the Member States of the EEA under the following names:
Austria, Belgium, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Luxemburg, Netherlands, Portugal, Spain, Sweden, UK:
TRUSOPT
This leaflet was last revised in June 2015.
How can I learn more about TRUSOPT, increased eye pressure or glaucoma?
This leaflet gives you the most important information about TRUSOPT. If you have any questions after you have read it, ask your doctor or pharmacist, who can give you more information about TRUSOPT and your eye condition.
Further information about glaucoma is available from:
International Glaucoma Association (IGA)
Woodcote House
15 Highpoint Business Village
Henwood,
Ashford Kent, TN24 8DH Tel: 01233 648170
Registered Charity number 274681.
Alternatively, if you or someone you know has problems with their vision, and you require further advice or information, please phone the Royal National Institute for the Blind (RNIB) Helpline on 0303 123 9999, Monday to Friday 8.45am to 5.30pm, calls charged at local rate.
(The IGA and RNIB are independent UK charities and are not associated with Santen Oy.)
Santen Oy, Niittyhaankatu 20, 33720 Tampere, Finland.