Vagifem 10 Micrograms Vaginal Tablets
Out of date information, search another03
8-2791-01-001-5_v1-2:Layout 1 2014-04-10
7:34 AM
Page
Regulatory Operations
Insert size: 210x297-023 Current: 3.0
Colour: PMS 280C

Compare
Women aged 50 to 79 who are not taking HRT, on average, 9 to 17 in 1,000 will be diagnosed with breast cancer over a 5-year period. For women aged 50 to 79 who are taking oestrogen-progestagen
HRT over 5 years, there will be 13 to 23 cases in 1,000 users (i.e. an extra 4 to 6 cases).
novo nordisk*
PACKAGE LEAFLET: INFORMATION FOR THE USER
Vagifem®
10 micrograms vaginal tablets
Estradiol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Vagifem® is and what it is used for
2. What you need to know before you use Vagifem®
3. How to use Vagifem®
4. Possible side effects
5. How to store Vagifem®
6. Contents of the pack and other information
1. What Vagifem® is and what it is used for
Vagifem® contains estradiol
• Estradiol is a female sex hormone.
• It belongs to a group of hormones called oestrogens.
• It is exactly the same as the estradiol produced by the ovaries of women.
Vagifem® belongs to a group of medicines called local Hormone Replacement Therapy (HRT).
It is used to relieve menopausal symptoms in the vagina such as dryness or irritation. In medical terms this is known as 'vaginal atrophy'. It is caused by a drop in the levels of oestrogen in your body. This happens naturally after the menopause.
Vagifem® works by replacing the oestrogen which is normally produced in the ovaries of women. It is inserted into your vagina, so the hormone is released where it is needed. This may relieve discomfort in the vagina.
The experience of treating women older than 65 years is limited.
2. What you need to know before you use Vagifem®
Medical history and regular check-ups
The use of HRT carries risks which need to be considered when deciding whether to start taking it, or whether to carry on taking it.
Before you start (or restart) HRT, your doctor will ask about your own and your family's medical history. Your doctor may decide to perform a physical examination. This may include an examination of your breasts and/or internal examination, if necessary.
Go for regular breast screening as recommended by your doctor.
Do not use Vagifem®:
If any of the following apply to you. If you are not sure about any of the points below, talk to your doctor
before using Vagifem®.
Do not use Vagifem® if
• You are allergic (hypersensitive) to estradiol or any of the other ingredients of Vagifem® (listed in section 6 'Contents of the pack and other information').
• You have or have ever had breast cancer, or you are suspected of having it.
• You have or have ever had cancer which is sensitive to oestrogens, such as cancer of the womb lining (endometrium), or you are suspected of having it.
• You have any unexplained vaginal bleeding.
• You have excessive thickening of the womb lining (endometrial hyperplasia) that is not being treated.
• You have or have ever had a blood clot in a vein
(thrombosis), such as in the legs (deep venous thrombosis) or the lungs (pulmonary embolism).
• You have a blood clotting disorder (such as protein C, protein S or antithrombin deficiency).
• You have or have recently had
a disease caused by blood clots in the arteries, such as a heart attack, stroke or angina.
• You have or have ever had a liver disease and your liver function tests have not returned to normal.
• You have a rare blood problem called 'porphyria', which is passed down in families (inherited).
If any of the above conditions appear for the first time while using Vagifem®, stop using it at once and consult your doctor immediately.
Warnings and precautions
Tell your doctor if you have or have ever had any of the following problems before you start the treatment. If so, you should see your doctor more often for check-ups.
Vagifem®, as opposed to systemic oestrogen, is for local treatment in the vagina, and the absorption into the blood is very low. It is therefore less likely that the conditions mentioned below will get worse or come back during treatment with Vagifem®.
• Asthma
• Epilepsy
• Diabetes
• Gallstones
• High blood pressure
• Migraines or severe headaches
• A liver disorder, such as a benign liver tumour
• Growth of womb lining outside your womb (endometriosis) or a history of excessive growth of the womb lining (endometrial hyperplasia)
• A disease affecting the eardrum and hearing (otosclerosis)
• A disease of the immune system that affects many organs of the body (systemic lupus erythematosus, SLE)
• Increased risk of getting an oestrogen-sensitive cancer (such as having a mother, sister or grandmother who has had breast cancer)
• Increased risk of developing blood clots (see 'Blood clots in a vein (thrombosis)')
• Fibroids inside your womb
• A very high level of fat in your blood (triglycerides)
• Fluid retention due to cardiac or kidney problems.
Stop using Vagifem® and see a doctor immediately
If you notice any of the following when using HRT:
• Migraine-like headaches which happen for the first time
• Yellowing of your skin or the whites of your eyes (jaundice). These may be signs of a liver disease
• A large rise in your blood pressure (symptoms may be headache, tiredness, dizziness)
• Any of the conditions mentioned in the 'Do not use Vagifem®' section
• If you become pregnant
• If you notice signs of a blood clot, such as:
- painful swelling and redness of the legs
- sudden chest pain
- difficulty in breathing.
For more information, see 'Blood clots in a vein (thrombosis)'.
The following risks apply to HRT medicines which circulate in the blood. It is not known how these risks apply to locally administered treatments such as Vagifem®.
HRT and cancer
Excessive thickening of the lining of the womb (endometrial hyperplasia) and cancer of the lining of the womb (endometrial cancer)
Taking oestrogen-only HRT tablets for a long time can increase the risk of developing cancer of the womb lining (the endometrium). It is uncertain whether long term (more than one year) or repeated use of local vaginally administered oestrogen products possess a similar risk. Vagifem® has been shown to have very low systemic absorption initially during treatment, and the addition of a progestagen is therefore not necessary.
If you get breakthrough bleeding or spotting, it's usually nothing to worry about, but you should make an appointment to see your doctor. It could be a sign that your endometrium has become thicker.
Compare
In women who still have a womb and who are not taking HRT, on average, 5 in 1,000 will be diagnosed with endometrial cancer between the ages of 50 and 65.
For women aged 50 to 65 who still have a womb and who take oestrogen-only HRT, between 10 and 60 women in 1,000 will be diagnosed with endometrial cancer (i.e. between 5 and 55 extra cases), depending on the dose and for how long it is taken.
Breast cancer
Evidence suggests that taking combined oestrogen-progestagen and possibly also oestrogen-only HRT increases the risk of breast cancer. The extra risk depends on how long you take HRT. The additional risk becomes clear within a few years. However, it returns to normal within a few years (at most 5) after stopping treatment.
For women who have had their womb removed and who are using oestrogen-only HRT for 5 years, little or no increase in breast cancer risk is shown.
Regularly check your breasts. See your doctor if you notice any changes such as:
• dimpling of the skin
• changes in the nipple
• any lumps you can see or feel.
Ovarian cancer
Ovarian cancer is rare. A slightly increased risk of ovarian cancer has been reported in women taking HRT for at least 5 to 10 years.
Compare
Women aged 50 to 69 who are not taking HRT, on average, about 2 women in 1,000 will be diagnosed with ovarian cancer over a 5-year period. For women who have been taking HRT for 5 years, there will be between 2 and 3 cases per 1,000 users (i.e. up to 1 extra case).
Effect of HRT on heart and circulation
Blood clots in a vein (thrombosis) The risk of blood clots in the veins
is about 1.3- to 3- times higher in HRT users than in non-users, especially during the first year of taking it. Blood clots can be serious, and if one travels to the lungs, it can cause chest pain, breathlessness, fainting or even death.
You are more likely to get a blood clot in your veins as you get older and if any of the following apply to you. Inform your doctor if any of these situations apply to you:
• you are unable to walk for a long time because of major surgery, injury or illness
• you are seriously overweight (BMI >30 kg/m2)
• you have any blood clotting problem that needs long-term treatment with a medicine used to prevent blood clots
• if any of your close relatives has ever had a blood clot in the leg, lung or another organ
• you have systemic lupus erythematosus (SLE)
• you have cancer.
For signs of a blood clot, see 'Stop using Vagifem® and see a doctor immediately'.
Compare
Looking at women in their 50s who are not taking HRT, on average, over a 5-year period, 4 to 7 in 1,000 would be expected to get a blood clot in a vein.
For women in their 50s who have been taking oestrogen-progestagen HRT for over 5 years, there will be 9 to 12 cases in 1,000 users (i.e. 5 extra cases)
For women in their 50s who have had their womb removed and have been taking oestrogen-only HRT for over 5 years, there will be 5 to 8 cases in 1,000 users (i.e. 1 extra case).
Heart disease (heart attack)
There is no evidence that HRT will prevent a heart attack.
Women over the age of 60 years who use oestrogen-progestagen HRT are slightly more likely to develop heart disease than those not taking any HRT.
For women who have had their womb removed and are taking oestrogen-only therapy there is no increased risk of developing a heart disease.
ON
00

t

QJ QJ -
~o ~o
o o u u .
0s
O
O

u E
QJ L
-i= <Ti
2
8 1
8-2791-01-001-5_v1-2:Layout 1 2014-04-10
7:34 AM
Page
Code start
Code: 100% Direction —► Length: Max. 29 mm (100%)
Compare
Looking at women in their 50s who are not taking HRT, on average, 8 in 1,000 would be expected to have a stroke over a 5-year period. For women in their 50s who are taking HRT, there will be 11 cases in 1,000 users, over 5 years (i.e. 3 extra cases).
3. How to use Vagifem®

Stroke
The risk of getting stroke is about 1.5-times higher in HRT users than in non-users. The number of extra cases of stroke due to use of HRT will increase with age.
Other conditions
HRT will not prevent memory loss. There is some evidence of a higher risk of memory loss in women who start using HRT after the age of 65. Speak to your doctor for advice.
Other medicines and Vagifem®
Please tell your doctor or pharmacist if you are using or have recently used any other medicines, including medicines obtained without a prescription. However, Vagifem® is not likely to affect other medicines. This is because Vagifem® is used for a local treatment in the vagina and contains a very low dose of estradiol.
Pregnancy and breast-feeding
Vagifem® is for use in postmenopausal women only. If you become pregnant, stop using Vagifem® and contact your doctor.
Driving and using machines
No known effect.
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Using this medicine
• You can start using Vagifem® on any day which is best for you.
• Insert the vaginal tablet into your vagina with the applicator.
The 'USER INSTRUCTIONS' at the end of the leaflet tell you how to do this. Read the instructions carefully before using Vagifem®.
How much to use
• Use one vaginal tablet each day for the first 2 weeks.
• Then use one vaginal tablet twice a week. Leave 3 or 4 days between each dose.
General information about treating symptoms of the menopause
• When using medicines for any menopausal symptoms, it is recommended to use the lowest dose that works, and to use the medicine for as short a time as it is needed.
• Treatment should only be continued if the benefit is greater than the risk. Talk to your doctor about this.
If you use more Vagifem® than
you should
• If you have used more Vagifem® than you should, talk to a doctor or pharmacist.
• Vagifem® is for local treatment inside the vagina. The dose of estradiol is so low that
a considerable number of tablets would have to be taken to approach the dose normally used for treatment taken by mouth.
If you forget to use Vagifem®
• If you forget a dose, use the medicine as soon as you remember.
• Do not use a double dose to make up for a forgotten dose.
If you stop using Vagifem®
Do not stop using Vagifem® without talking to your doctor. Your doctor will explain the effects of stopping treatment. He or she will also discuss other possibilities for treatment with you.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common
• Headache
• Stomach pain
• Vaginal bleeding, discharge or discomfort.
Uncommon
• An infection of the genitals caused by a fungus
• Feeling sick (nausea)
• Rash
• Weight increase
• Hot flush
• Hypertension.
Very rare
• Diarrhoea
• Fluid retention
• Migraine aggravated
• Generalised hypersensitivity (e.g. anaphylactic reaction/shock).
The frequency of possible side effects listed above is defined using the following convention:
Very common (affects more than 1 user in 10)
Common (affects 1 to 10 users in 100)
Uncommon (affects 1 to 10 users in 1,000)
Rare (affects 1 to 10 users in 10,000) Very rare (affects less than 1 user in 10,000)
Not known (frequency cannot be estimated from the available data).
The following side effects can occur with systemic oestrogen treatment:
• Gall bladder disease
• Various skin disorders:
- discoloration of the skin especially of the face or neck known as 'pregnancy patches' (chloasma)
- painful reddish skin nodules (erythema nodosum)
- rash with target-shaped reddening or sores (erythema multiforme).
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme Website: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Vagifem®
Keep this medicine out of the sight and reach of children.
Do not refrigerate.
Do not use this medicine after the expiry date which is stated on the carton label and blister after EXP.
The expiry date refers to the last day of that month.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
This medicine may cause risk to the aquatic environment.
6. Contents of the pack and other information
What Vagifem® contains
• The active substance is estradiol 10 micrograms (as estradiol hemihydrate). Each vaginal tablet contains 10 micrograms estradiol (as estradiol hemihydrate).
• Other ingredients are: hypromellose, lactose monohydrate, maize starch and magnesium stearate.
• The film-coating contains: hypromellose and macrogol 6000.
What Vagifem® looks like and contents of the pack
Each white vaginal tablet comes in an applicator which is used once only.
Vagifem® is engraved with NOVO 278 on one side.
Pack sizes:
18 vaginal tablets with applicators. 24 vaginal tablets with applicators.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Novo Nordisk A/S Novo Alle
DK-2880 Bagsvsrd Denmark
The registered office in the UK is:-
Novo Nordisk Limited
3 City Place
Beehive Ring Road
Gatwick
West Sussex
RH6 0PA
Tel: (01293) 613555
This leaflet was last revised in: 06/2014
USER INSTRUCTIONS How to use Vagifem®
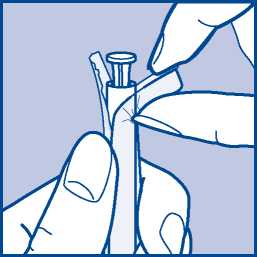
1. Tear off one single blister pack. Open the end as shown in the picture.
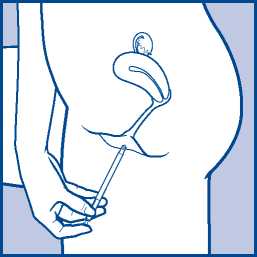
2. Insert the applicator carefully into the vagina.
Stop when you can feel some resistance (8-10 cm).
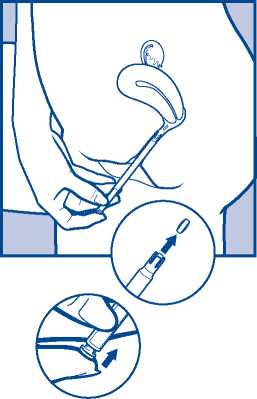
3. To release the tablet, gently press the push-button until you feel a click. The tablet will stick to the wall of the vagina straight away.
It will not fall out if you stand up or walk.
4. Take out the applicator and throw it away.
Vagifem® is a registered trademark owned by Novo Nordisk FemCare AG, Switzerland
© 2014
Novo Nordisk A/S
novo nordisk